5.3 Erythrocytes
Leanne Dooley
Learning Objectives
By the end of this section, you will be able to:
- Describe the anatomy of erythrocytes
- Discuss the various steps in the lifecycle of an erythrocyte
- Explain the composition and function of haemoglobin
The erythrocyte, commonly known as a red blood cell (or RBC), is by far the most common formed element: A single drop of blood contains millions of erythrocytes and just thousands of leukocytes. Specifically, males have about 5.4 million erythrocytes per microlitre (µL) of blood, and females have approximately 4.8 million per µL. In fact, erythrocytes are estimated to make up about 25 percent of the total cells in the body. As you can imagine, they are quite small cells, with a mean diameter of only about 7–8 micrometres (µm) (Figure 5.3.1). The primary functions of erythrocytes are to pick up inhaled oxygen from the lungs and transport it to the body’s tissues, and to pick up some (about 24 percent) carbon dioxide waste at the tissues and transport it to the lungs for exhalation. Erythrocytes remain within the vascular network. Although leukocytes typically leave the blood vessels to perform their defensive functions, movement of erythrocytes from the blood vessels is abnormal.
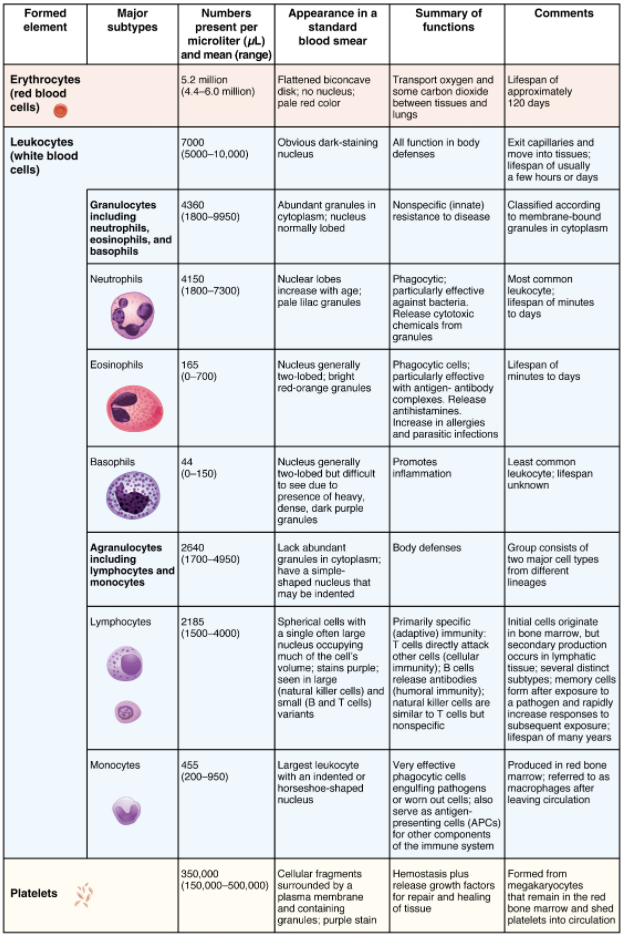
Shape and Structure of Erythrocytes
As an erythrocyte matures in the red bone marrow, it extrudes its nucleus and most of its other organelles. During the first day or two that it is in the circulation, an immature erythrocyte, known as a reticulocyte, will still typically contain remnants of organelles. Reticulocytes should comprise approximately 1–2 percent of the erythrocyte count and provide a rough estimate of the rate of RBC production, with abnormally low or high rates indicating deviations in the production of these cells. These remnants, primarily of networks (reticulum) of ribosomes, are quickly shed, however, and mature, circulating erythrocytes have few internal cellular structural components. Lacking mitochondria, for example, they rely on anaerobic respiration. This means that they do not utilise any of the oxygen they are transporting, so they can deliver it all to the tissues. They also lack endoplasmic reticula and do not synthesise proteins. Erythrocytes do, however, contain some structural proteins that help the blood cells maintain their unique structure and enable them to change their shape to squeeze through capillaries. This includes the protein spectrin, a cytoskeletal protein element. They also contain enzymes that protect haemoglobin from oxidisation.
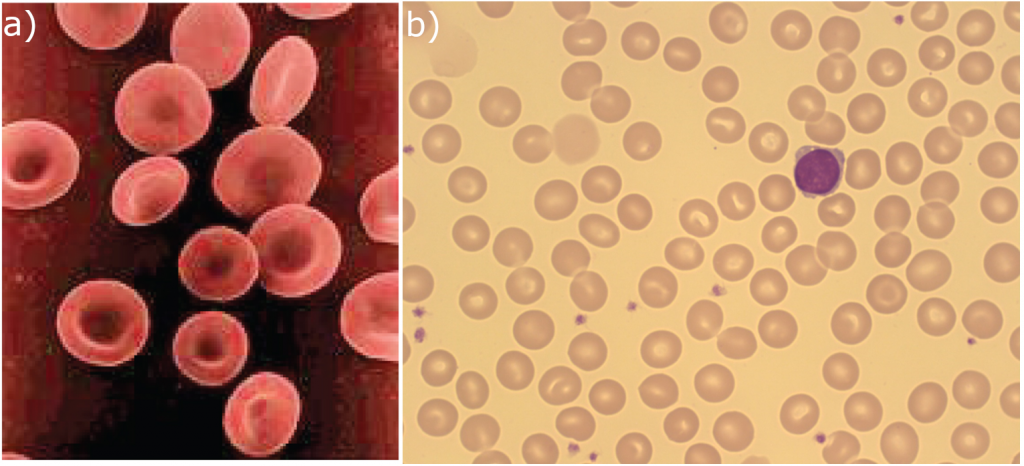
Erythrocytes are biconcave disks; that is, they are plump at their periphery and very thin in the centre (Figure 5.3.2). Since they lack most organelles, there is more interior space for the presence of the haemoglobin molecules that, as you will see shortly, transport gases. The biconcave shape also provides a greater surface area across which gas exchange can occur, relative to its volume; a sphere of a similar diameter would have a lower surface area-to-volume ratio. In the capillaries, the oxygen carried by the erythrocytes can diffuse into the plasma and then through the capillary walls to reach the cells, whereas some of the carbon dioxide produced by the cells as a waste product diffuses into the capillaries to be picked up by the erythrocytes. Capillary beds are extremely narrow, slowing the passage of the erythrocytes and providing an extended opportunity for gas exchange to occur. However, the space within capillaries can be so minute that, despite their own small size, erythrocytes may have to fold in on themselves if they are to make their way through. Fortunately, their structural proteins like spectrin are flexible, allowing them to bend over themselves to a surprising degree, then spring back again when they enter a wider vessel. In wider vessels, erythrocytes may stack up much like a roll of coins, forming a rouleaux, from the French word for “roll.”
Haemoglobin
Haemoglobin is a large molecule made up of proteins and iron. It consists of four folded chains of a protein called globin, designated alpha 1 and 2, and beta 1 and 2 (Figure 5.3.3a). Each of these globin molecules is bound to a red pigment molecule called haem, which contains an ion of iron (Fe2+) (Figure 5.3.3b).
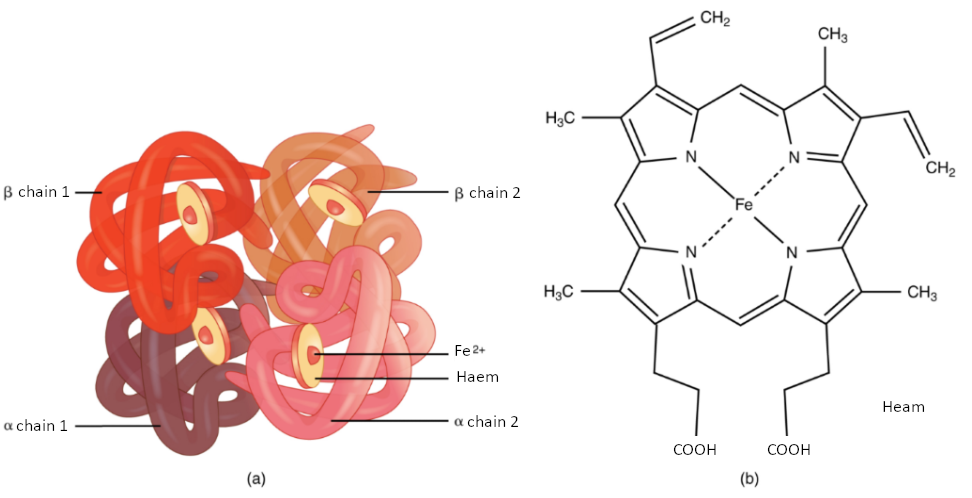
Each iron ion in the haem can bind to one oxygen molecule; therefore, each haemoglobin molecule can transport four oxygen molecules. An individual erythrocyte may contain about 300 million haemoglobin molecules and therefore can bind to and transport up to 1.2 billion oxygen molecules (see Figure 5.3.3b).
In the lungs, haemoglobin picks up oxygen, which binds to the iron ions, forming oxyhaemoglobin. The bright red, oxygenated haemoglobin travels to the body tissues, where it releases some of the oxygen molecules, becoming darker red deoxyhemoglobin, sometimes referred to as reduced haemoglobin. Oxygen release depends on the need for oxygen in the surrounding tissues, so haemoglobin rarely if ever leaves all its oxygen behind. In the capillaries, carbon dioxide enters the bloodstream. About 76 percent dissolves in the plasma, some of it remaining as dissolved CO2, and the remainder forming bicarbonate ion. About 23–24 percent of it binds to the amino acids in haemoglobin, forming a molecule known as carbaminohemoglobin. From the capillaries, the haemoglobin carries carbon dioxide back to the lungs, where it releases it for exchange of oxygen.
Changes in the levels of RBCs can have significant effects on the body’s ability to effectively deliver oxygen to the tissues. Ineffective haematopoiesis results in insufficient numbers of RBCs and results in one of several forms of anaemia. An overproduction of RBCs produces a condition called polycythaemia. The primary drawback with polycythaemia is not a failure to directly deliver enough oxygen to the tissues, but rather the increased viscosity of the blood, which makes it more difficult for the heart to circulate the blood and increases the risk of thrombosis (excessive blood clot formation).
In patients with insufficient haemoglobin, the tissues may not receive sufficient oxygen, resulting in another form of anaemia. In determining oxygenation of tissues, the value of greatest interest in healthcare is the percent saturation; that is, the percentage of haemoglobin sites occupied by oxygen in a patient’s blood. Clinically this value is commonly referred to simply as “percent sat.”
Percent saturation is normally monitored using a device known as a pulse oximeter, which is applied to a thin part of the body, typically the tip of the patient’s finger. The device works by sending two different wavelengths of light (one red, the other infrared) through the finger and measuring the light with a photodetector as it exits. Haemoglobin absorbs light differentially depending upon its saturation with oxygen. The machine calibrates the amount of light received by the photodetector against the amount absorbed by the partially oxygenated haemoglobin and presents the data as percent saturation. Normal pulse oximeter readings range from 95–100 percent. Lower percentages reflect hypoxaemia, or low blood oxygen. The term hypoxia is more generic and simply refers to low oxygen levels. Oxygen levels are also directly monitored from free oxygen in the plasma typically following an arterial stick. When this method is applied, the amount of oxygen present is expressed in terms of partial pressure of oxygen or simply pO2 and is typically recorded in units of millimetres of mercury, mm Hg.
The kidneys filter about 180 litres (~380 pints) of blood in an average adult each day and thus serve as ideal sites for receptors that determine oxygen saturation. In response to hypoxaemia, less oxygen will exit the vessels supplying the kidney, resulting in hypoxia (low oxygen concentration) in the tissue fluid of the kidney where oxygen concentration is monitored. Interstitial fibroblasts within the kidney secrete EPO, thereby increasing erythrocyte and haemoglobin production and restoring oxygen levels. In a classic negative-feedback loop, as oxygen saturation rises, EPO secretion falls, and vice versa, thereby maintaining homeostasis. Populations dwelling at high altitudes, with inherently lower levels of oxygen in the atmosphere, naturally maintain a haematocrit higher than people living at sea level. Consequently, people traveling to high altitudes may experience symptoms of hypoxaemia, such as fatigue, headache, and shortness of breath, for a few days after their arrival. In response to the hypoxaemia, the kidneys secrete EPO to step up the production of erythrocytes until homeostasis is achieved once again. To avoid the symptoms of hypoxaemia, or altitude sickness, mountain climbers typically rest for several days to a week or more at a series of camps situated at increasing elevations to allow EPO levels and, consequently, erythrocyte counts to rise. When climbing the tallest peaks, such as Mt. Everest and K2 in the Himalayas, many mountain climbers rely upon bottled oxygen as they near the summit.
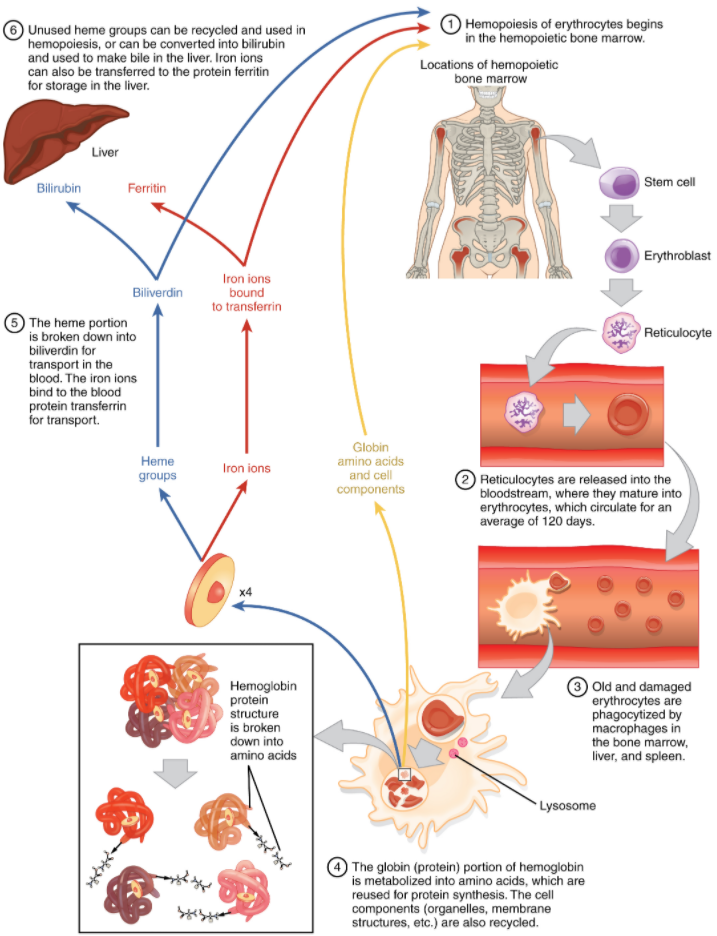
Lifecycle of Erythrocytes
Production of erythrocytes in the marrow occurs at the staggering rate of more than 2 million cells per second. For this production to occur, several raw materials must be present in adequate amounts. These include the same nutrients that are essential to the production and maintenance of any cell, such as glucose, lipids, and amino acids. However, erythrocyte production also requires several micronutrients including trace elements and vitamins:
- Iron. We have said that each haem group in a haemoglobin molecule contains an ion of the trace mineral iron. On average, less than 20 percent of the iron we consume is absorbed. Haem iron, from animal foods such as meat, poultry, and fish, is absorbed more efficiently than non-haem iron from plant foods. Upon absorption, iron becomes part of the body’s total iron pool. The bone marrow, liver, and spleen can store iron in the protein compounds ferritin and haemosiderin. Ferroprotein transports the iron across the intestinal cell plasma membranes and from its storage sites into tissue fluid where it enters the blood. When EPO stimulates the production of erythrocytes, iron is released from storage, bound to transferrin, and carried to the red marrow where it attaches to erythrocyte precursors.
- Copper. A trace mineral, copper is a component of two plasma proteins, hephaestin and ceruloplasmin. Without these, haemoglobin could not be adequately produced. Located in intestinal villi, hephaestin enables iron to be absorbed by intestinal cells. Ceruloplasmin transports copper. Both enable the oxidation of iron from Fe2+to Fe3+, a form in which it can be bound to its transport protein, transferrin, for transport to body cells. In a state of copper deficiency, the transport of iron for haem synthesis decreases, and iron can accumulate in tissues, where it can eventually lead to organ damage.
- Zinc. The trace mineral zinc functions as a co-enzyme that facilitates the synthesis of the haem portion of haemoglobin.
- B vitamins. The B vitamins folate and vitamin B12 function as co-enzymes that facilitate DNA synthesis. Thus, both are critical for the synthesis of new cells, including erythrocytes.
Erythrocytes live up to 120 days in the circulation, after which the worn-out cells are removed by a type of myeloid phagocytic cell called a macrophage, located primarily within the bone marrow, liver, and spleen. The components of the degraded erythrocytes’ haemoglobin are further processed as follows:
Globin, the protein portion of haemoglobin, is broken down into amino acids, which can be sent back to the liver for construction of new proteins.
The iron contained in the haem portion of haemoglobin may be stored in the liver or spleen, primarily in the form of ferritin or hemosiderin, or carried through the bloodstream by transferrin to the red bone marrow for recycling into new erythrocytes.
The non-iron portion of haem is degraded into the waste product biliverdin, a green pigment, and then into another waste product, bilirubin, a yellow pigment. Bilirubin binds to albumin and travels in the blood to the liver, which excretes it in bile, a fluid released into the intestines to help emulsify dietary fats. In the large intestine, bacteria break the bilirubin apart from the bile and convert it to urobilinogen and then into stercobilin. It is then eliminated from the body in the faeces. Broad-spectrum antibiotics typically eliminate these “good” bacteria as well and this may alter the colour of faeces. The kidneys also remove any circulating bilirubin and other related metabolic by-products such as urobilins and secrete them into the urine.
The breakdown pigments formed from the destruction of haemoglobin can be seen in a variety of situations. At the site of an injury, biliverdin from damaged RBCs produces some of the dramatic colours associated with bruising. With a failing liver, bilirubin cannot be removed effectively from circulation and causes the body to assume a yellowish tinge associated with jaundice. Stercobilins within the faeces produce the typical brown colour associated with this waste and the yellow of urine is associated with the urobilins.
The erythrocyte lifecycle is summarised in Figure 5.3.4.
Disorders of Erythrocytes
The size, shape, and number of erythrocytes, and the number of haemoglobin molecules can have a major impact on a person’s health. When the number of RBCs or amount of haemoglobin is deficient, the general condition is called anaemia. There are more than 400 types of anaemia and approximately 4.5% of Australian adults are at risk of anaemia. Anaemia can be broken down into three major groups: those caused by blood loss, those caused by faulty or decreased RBC production, and those caused by excessive destruction of RBCs. Clinicians often use two groupings in diagnosis: The kinetic approach focuses on evaluating the production, destruction, and removal of RBCs, whereas the morphological approach examines the RBCs themselves, paying particular emphasis to their size. A common test is the mean cell volume (MCV), which measures RBC size. Normal-sized cells are referred to as normocytic, smaller-than-normal cells are referred to as microcytic, and larger-than-normal cells are referred to as macrocytic. Reticulocyte counts are also important and may reveal inadequate production of RBCs. The effects of the various anaemias are widespread, because reduced numbers of RBCs or haemoglobin will result in lower levels of oxygen being delivered to body tissues. Since oxygen is required for tissue functioning, anaemia produces fatigue, lethargy, and an increased risk for infection. An oxygen deficit in the brain impairs the ability to think clearly and may prompt headaches and irritability. Lack of oxygen leaves the patient short of breath, even as the heart and lungs work harder in response to the deficit.
Anaemias resulting from acute blood loss are usually easy to identify. In addition to bleeding from external wounds or other visible lesions, blood loss anaemias may be due to chronic bleeding from ulcers, haemorrhoids, inflammation of the stomach (gastritis) and some cancers of the gastrointestinal tract. The excessive use of aspirin or other nonsteroidal anti-inflammatory drugs such as ibuprofen can trigger ulceration and gastritis leading to chronic blood loss. Excessive menstruation and loss of blood during childbirth are also potential causes of anaemia.
Anaemias caused by faulty or decreased RBC production include sickle cell anaemia, iron deficiency, vitamin deficiency anaemia and diseases of the bone marrow and stem cells.
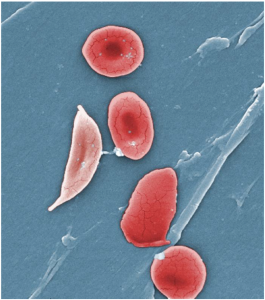
A characteristic change in the shape of erythrocytes is seen in sickle cell disease (also referred to as sickle cell anaemia). Sickle cell anaemia is caused by production of an abnormal type of haemoglobin, called haemoglobin S, which delivers less oxygen to tissues and causes erythrocytes to assume a sickle (or crescent) shape, especially at low oxygen concentrations (Figure 5.3.5). These abnormally shaped cells can then become lodged in narrow capillaries because they are unable to fold in on themselves to squeeze through, blocking blood flow to tissues and causing a variety of serious problems from painful joints to delayed growth and even blindness and cerebrovascular accidents (strokes). Sickle cell anaemia is a genetic or inherited blood disorder more commonly found in individuals of African, Middle Eastern, Asian, Indian and Mediterranean descent.
Iron deficiency anaemia is the most common type of anaemia and results when the amount of available iron is insufficient to allow production of sufficient haemoglobin. This condition can occur in individuals with a deficiency of iron in the diet and is especially common in teens and children as well as in vegans and vegetarians. Additionally, iron deficiency anaemia may be caused by either an inability to absorb and transport iron, or slow chronic bleeding usually from the gastrointestinal tract or through heavy menstruation.
Vitamin-deficient anaemias generally involve insufficient vitamin B12 and folate.
Megaloblastic anaemia is the result of a deficiency of vitamin B12 and/or folate, and often involves diets deficient in these essential nutrients. Lack of animal products or viable alternate sources in the diet can lead to a lack of vitamin B12 and overcooking or eating insufficient amounts of vegetables may lead to a lack of folate.
Pernicious anaemia is a type of megaloblastic anaemia caused by poor absorption of vitamin B12 and is often seen in patients with Crohn’s disease (a severe intestinal disorder often treated by surgery), surgical removal of the intestines or stomach (common in some weight loss surgeries), the presence of intestinal parasites and AIDS.
Pregnancies, some medications, excessive alcohol consumption, and some diseases such as coeliac disease are also associated with vitamin deficiencies. It is essential to provide sufficient folic acid during the early stages of pregnancy to reduce the risk of neurological defects, including spina bifida, a failure of the neural tube to close.
Assorted disease processes can also interfere with the production and formation of RBCs and haemoglobin. If myeloid stem cells are defective or replaced by cancer cells, there will be insufficient quantities of RBCs produced.
Aplastic anaemia is the condition in which there are deficient numbers of RBC stem cells. Aplastic anaemia is often inherited, or it may be triggered by radiation, medication, chemotherapy, or infection, particularly by Parvo virus B19.
Thalassaemia is an inherited condition typically occurring in individuals from the Middle East, the Mediterranean, Africa, and Southeast Asia, in which the lifespan of RBCs is shortened due to unbalanced production of globin chains.
Hereditary spherocytosis is another inherited form of anaemia associated with shortened RBC survival. In this case the spectrin in the RBC membrane is defective resulting in loss of membrane without loss of cell contents as the cells circulate through small capillaries. This change in the surface area to volume ratio causes the RBCs to lose their bi-concave disc shape and become spherical (spherocytes). Spherocytes are less flexible than normal RBC and become trapped in the spleen, where they are removed by splenic macrophages.
Lead exposure from industrial sources or even dust from paint chips of iron-containing paints or pottery that has not been properly glazed may also lead to destruction of the red marrow.
Various disease processes can also lead to anaemias. These include chronic kidney diseases often associated with decreased production of EPO, hypothyroidism, some forms of cancer, especially leukaemia and bone cancers, and autoimmune diseases such as lupus, and rheumatoid arthritis.
In contrast to anaemia, an elevated RBC count is called polycythaemia and is detected in a patient’s elevated haematocrit. It can occur transiently in a person who is dehydrated; when water intake is inadequate or water losses are excessive, the plasma volume falls. As a result, the haematocrit rises. For reasons mentioned earlier, a mild form of polycythaemia is chronic but normal in people living at high altitudes. Some elite athletes train at high altitudes specifically to induce this phenomenon. Finally, a type of bone marrow disease called polycythaemia vera (from the Greek vera = “true”) causes an excessive production of immature erythrocytes. Polycythaemia vera can dangerously elevate the viscosity of blood, raising blood pressure and making it more difficult for the heart to pump blood throughout the body. It is a relatively rare disease that occurs more often in men than women and is more likely to be present in people over 60 years of age.
Section Review
The most abundant formed elements in blood, erythrocytes, are red, biconcave disks packed with an oxygen-carrying compound called haemoglobin. The haemoglobin molecule contains four globin chains each bound to a pigment molecule called haem, which contains an ion of iron. In the bloodstream, iron picks up oxygen in the lungs and drops it off in the tissues; the amino acids in haemoglobin then transport carbon dioxide from the tissues back to the lungs. Erythrocytes live only 120 days on average, and thus must be continually replaced. Worn-out erythrocytes are phagocytosed by macrophages and their haemoglobin is broken down. The breakdown products are recycled or removed as wastes: Globin is broken down into amino acids for synthesis of new proteins; iron is stored in the liver or spleen or used by the bone marrow for production of new erythrocytes; and the remnants of haem are converted into bilirubin, or other waste products that are taken up by the liver and excreted in the bile or removed by the kidneys. Anaemia is a deficiency of RBCs and/or haemoglobin, whereas polycythaemia is an excess of RBCs.
Review Questions
Critical Thinking Questions
Click the drop down below to review the terms learned from this chapter.

