17 How and Why Do Cells Communicate?
In this section
Content in this section includes:
Social organisation is dependent on communication between the individuals that comprise that society; without communication, society would fall apart.
As with people, it is vital for individual cells to be able to interact with their environment. This is just as true for a one-celled organism growing in a water supply (such as Giardia parasites) and a large animal living in a desert (such as an emu or kangaroo). To properly respond to external stimuli, cells have developed complex mechanisms of communication that can receive a message, transfer the information across the plasma membrane and then produce changes within the cell in response to the message.
In multicellular organisms, cells send and receive chemical messages constantly to coordinate the actions of distant organs, tissues and cells.
The ability to send messages quickly and efficiently enables cells to coordinate and fine-tune their functions and this can even affect timing of reproduction.
While the necessity for cellular communication in larger organisms seems obvious, even single-celled organisms communicate with each other. Yeast cells signal each other to aid in finding other yeast cells for reproduction. Some forms of bacteria coordinate their actions to form large complexes called biofilms or to organise the production of toxins to remove competing organisms. The ability of cells to communicate through chemical signals originated in single cells and was essential for the evolution of multicellular organisms. The efficient and relatively error-free function of communication systems is vital for all life as we know it.
Unicellular and Multicellular Organisms
There are two kinds of communication in the world of living cells. Communication between cells is called intercellular signalling, and communication within a cell is called intracellular signalling. An easy way to remember the distinction is by understanding the Latin origin of the prefixes: inter- means “between” (for example, intersecting lines are those that cross each other) and intra- means “inside” (as in intravenous).
Chemical signals are released by signalling cells in the form of small, usually volatile or soluble molecules called ligands. A ligand is a molecule that binds another specific molecule, in some cases, delivering a signal in the process. Ligands can thus be thought of as chemical signalling molecules. Ligands interact with proteins in target cells, which are cells that are affected by chemical signals; these proteins are also called receptors. Ligands and receptors exist in several varieties; however, a specific ligand will have a specific receptor that typically binds only that ligand.
There are many ways to show the binding of a ligand to a specific receptor and this binding is typically represented with a very simple architecture (Figure 5.16) however the reality is much more complex (Figure 5.16). Ligand binding to receptor can be irreversible, reversible or very brief – however the ultimate outcome of ligand binding is an effect on the target cell. This effect can be a positive (agonist) effect or a negative (antagonist) effect. Also, some receptors for some ligands are found inside a cell (the signalling molecule can move through the cell membrane) and some are found on the outside of the cell membrane.
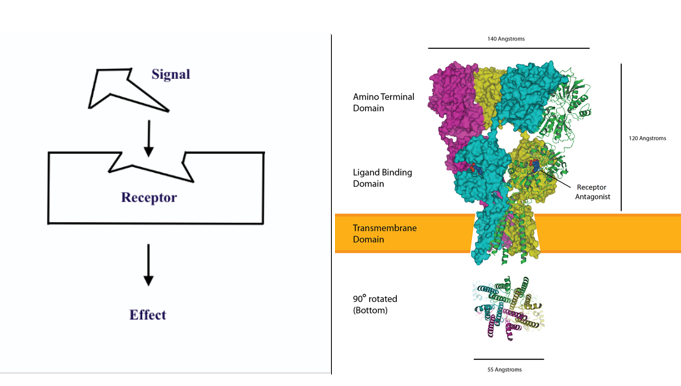
However…. The numbers of receptors on a cell are not static – they can be upregulated and also downregulated – and therefore the signal ‘strength’ can be modulated. In up-regulation, the number of receptors increases in response to increasing signal concentrations, making the cell more sensitive to the signal and allowing for more cellular activity. When the number of receptors decreases in response to increasing signal concentrations, called down-regulation, cellular activity is reduced. The modulation of receptor numbers can be seen in blood glucose control (with changes in insulin receptor numbers) and also with drugs (generally down-regulation of receptors so more drug is needed to achieve same effect as previously).
Unicellular Communication
Signalling in unicellular organisms, such as bacteria or protozoa, enables them to monitor extracellular conditions, ensure that there are sufficient amounts of nutrients in the local environment and allows them to move towards a better environment or away from hazardous environments. This movement, that is ‘informed’ by chemical signals in the environment, is known as chemotaxis.
There are also circumstances when unicellular microorganisms communicate with each other directly. The first evidence of bacterial communication was observed in a bacterium that has a symbiotic relationship with Hawaiian bobtail squid. When the population density of the bacteria reaches a certain level, specific gene expression is initiated and the bacteria produce bioluminescent proteins that emit light. Because the number of cells present in the environment (cell density) is the determining factor for signalling, bacterial signalling was named quorum sensing. In politics and business, a quorum is the minimum number of members required to be present to vote on an issue.
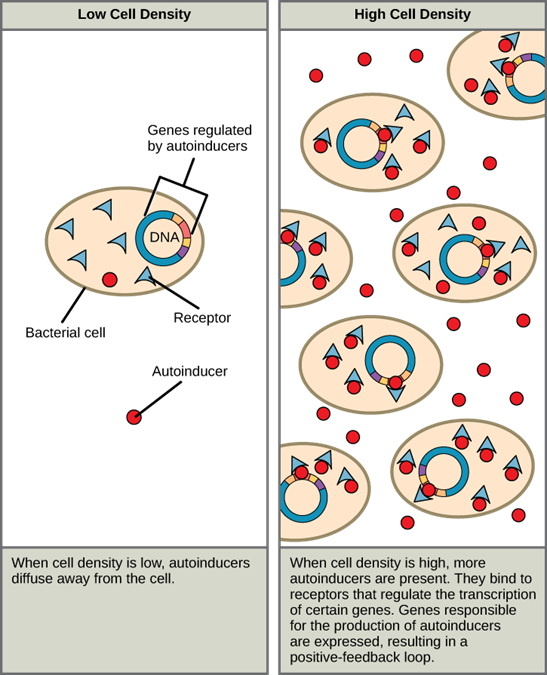
Quorum sensing uses autoinducers as signalling molecules. Autoinducers are signalling molecules secreted by bacteria to communicate with other bacteria of the same kind. The secreted autoinducers can be small, hydrophobic molecules, such as acyl-homoserine lactone (AHL), or larger peptide-based molecules; each type of molecule has a different mode of action. When AHL enters target bacteria, it binds to transcription factors, which then switch gene expression on or off. When the number of bacteria increases so does the concentration of the autoinducer, triggering increased expression of certain genes including autoinducers, which results in a self-amplifying cycle, also known as a positive feedback loop (Figure 5.17). The peptide autoinducers stimulate more complicated signalling pathways that include bacterial kinases. The changes in bacteria following exposure to autoinducers can be quite extensive. The pathogenic bacterium Pseudomonas aeruginosa has 616 different genes that respond to autoinducers!
Some species of bacteria that use quorum sensing also can form biofilms (Figure 5.18), which are complex colonies of bacteria (often containing several species) that exchange chemical signals to coordinate the release of toxins that will attack the host. Some of the biofilm bacteria can also transfer resistance genes to other bacteria and biofilms are more resistant to antimicrobials. As bacterial biofilms can sometimes be found on medical equipment; when biofilms invade implants such as hip or knee replacements or heart pacemakers, it is easy to see why they can cause life-threatening infections.
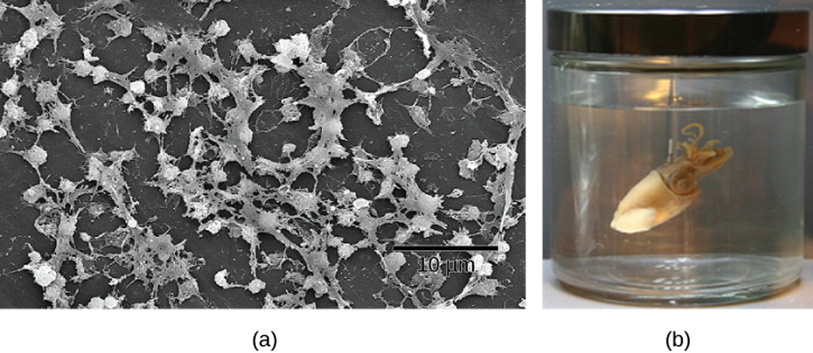
Biofilms
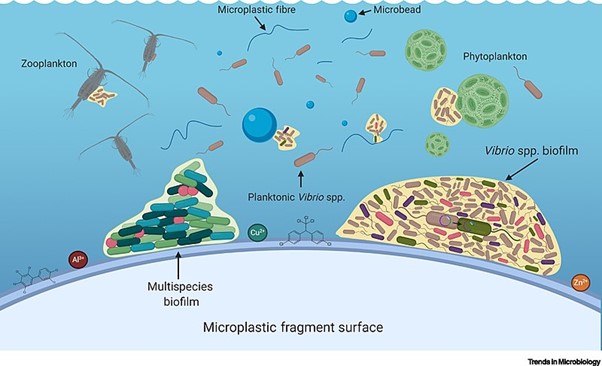
Biofilms are a complex aggregation of cells that are encased within an exocellular matrix and attached to a surface and fossil records show evidence of biofilm existence over 3.4 billion years ago. Biofilms can form on just about any surface, and are common in nature and industry, being found on the surfaces of rocks, caves, pipes, boat hulls, cooking vessels, and medical implants, for example. Importantly, biofilms can also form on environmental pollution such as microplastics (Figure 5.19).
The microbial community of a biofilm can be composed of one or two species but more commonly contains many different species of bacteria, each influencing the gene expression and growth of the others.
Biofilm Development
Despite the surface type, the basic steps for biofilm formation can be broken down into four steps:
- Cell disposition and attachment – for biofilm development to occur, free-floating or planktonic cells must collide with a suitable surface. Typically, the surface has been preconditioned with the deposits of environmental proteins and other molecules.
- Colonisation – cell-to-cell signalling occurs, leading to the expression of biofilm specific genes. These genes are associated with the communal production of extracellular polymeric substances. DNA released by some cells can be taken up by others, stimulating the expression of new genes.
- Maturation – the extracellular polymeric substance (EPS) matrix fully encases all the cells, as the biofilm continues to thicken and grow, forming a complex, dynamic community. Water channels form throughout the structure.
- Detachment and sloughing – individual cells or pieces of the biofilm are released to the environment, as a form of active dispersal. This release can be trigger by environmental factors, such as the concentration of nutrients or oxygen.
Cellular Advantages of Biofilms
Why do biofilms develop? There are certain advantages that cells enjoy while in a biofilm, over planktonic growth. Perhaps most importantly, biofilms offer cells increased protection from harmful conditions or substances, such as UV light, physical agitation, antimicrobial agents, and phagocytosis. It has been shown that bacteria within a biofilm are up to a thousand times more resistant to antibiotics than free-floating cells.
A biofilm also allows a cell population to “put down roots,” so that they can stay in close proximity to a nutrient-rich area – a biofilm that develops on a conduit pipe at a dairy plant will have continual access to fresh food, which is much better than being swept away with the final product.
Lastly, biofilms allow for cells to grow in microbial populations, where they can easily benefit from cell-to-cell communication and genetic exchange.
Biofilms and Human Health
The human body has many types of biofilms, some beneficial and some harmful. The layers of normal microbiota lining the intestinal and respiratory mucosa, for example, play a key role in protecting the body from infections by pathogens. However, other biofilms in the body can have a detrimental effect on health. The plaque that forms on teeth is a biofilm that can contribute to dental and periodontal disease. Biofilms can also form in wounds, sometimes causing serious infections that can spread. The bacterium Pseudomonas aeruginosa often colonises biofilms in the airways of patients with cystic fibrosis, causing chronic and sometimes fatal infections of the lungs. Biofilms can also form on medical devices used in or on the body, causing infections in patients with in-dwelling catheters, artificial joints, or contact lenses.
Pathogens embedded within biofilms exhibit a higher resistance to antibiotics than their free-floating counterparts. Cells in the deep layers of a biofilm are metabolically inactive and may be less susceptible to the action of antibiotics that disrupt metabolic activities. The EPS may also slow the diffusion of antibiotics and other antimicrobials, preventing them from reaching cells in the deeper layers of the biofilm. Phenotypic changes may also contribute to the increased resistance exhibited by bacterial cells in biofilms. The increased production of efflux pumps, membrane-embedded proteins that actively extrude antibiotics out of bacterial cells, have been shown to be an important mechanism of antibiotic resistance among biofilm-associated bacteria. Finally, biofilms provide an ideal environment for the exchange of extrachromosomal DNA, which often includes genes that confer antibiotic resistance.
Multicellular (Human) Communication
In multicellular organisms, there are four main categories of chemical signalling: autocrine signalling, direct signalling across gap junctions, paracrine signalling, endocrine signalling, (Figure 5.20). The main difference between the different categories of signalling is the distance that the signal travels through the organism to reach the target cell. It is notable that not all cells are affected by the same signals as each target cell must have the specific receptor available for the chemical signal (ligand) to bind.
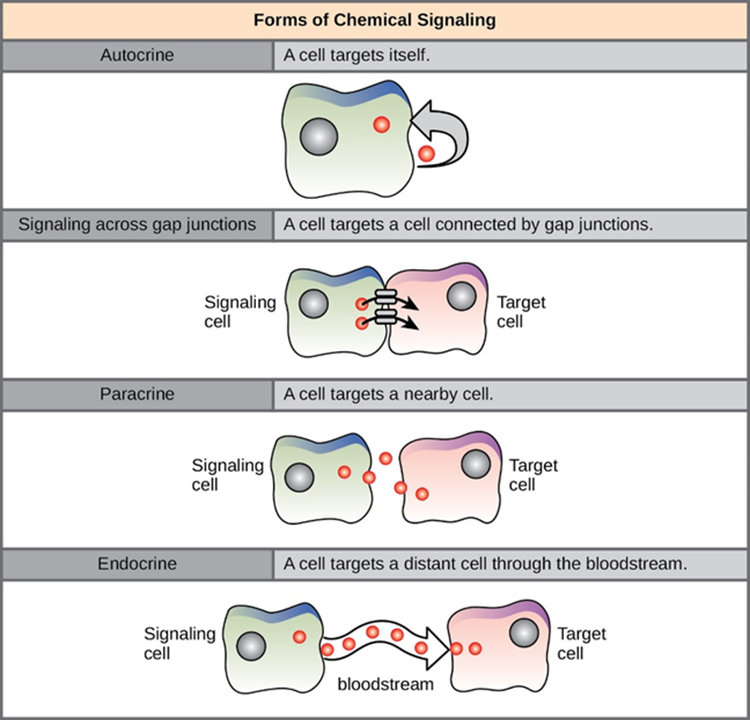
Forms of Chemical Signalling
Autocrine
Autocrine signals are produced by signalling cells that can also bind to the ligand that is released. This means the signalling cell and the target cell can be the same or a similar cell (the prefix auto- means self, a reminder that the signalling cell sends a signal to itself). This type of signalling often occurs during the early development of an organism to ensure that cells develop into the correct tissues and take on the proper function. Autocrine signalling also helps regulates pain sensation and inflammatory responses. Further, if a cell is infected with a virus, the cell can signal itself to undergo programmed cell death, killing the virus in the process. In some cases, neighbouring cells of the same type are also influenced by the released ligand. In embryological development, this process of stimulating a group of neighbouring cells may help to direct the differentiation of identical cells into the same cell type, thus ensuring the proper developmental outcome.
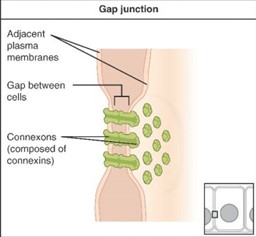
Gap Junctions
Gap junctions in animals and plasmodesmata in plants are connections between the plasma membranes of neighbouring cells (Figure 5.21). These fluid-filled channels allow small signalling molecules, called intracellular mediators, to diffuse between the two cells. Small molecules or ions, such as calcium ions (Ca2+), are able to move between cells, but large molecules like proteins and DNA cannot fit through the channels. The specificity of the channels ensures that the cells remain independent but can quickly and easily transmit signals. The transfer of signalling molecules communicates the current state of the cell that is directly next to the target cell; this allows a group of cells to coordinate their response to a signal that only one of them may have received. In plants, plasmodesmata are ubiquitous, making the entire plant into a giant communication network.
Paracrine
Signals that act locally between cells that are close together are called paracrine signals. Paracrine signals move by diffusion through the extracellular matrix. These types of signals usually elicit quick responses that last only a short period of time. In order to keep the response localised, paracrine ligand molecules are normally quickly degraded by enzymes or removed by neighbouring cells. Removing the signals will re-establish the concentration gradient for the signal, allowing them to quickly diffuse through the intracellular space if released again. One example of paracrine signalling is the transfer of signals across synapses between nerve cells via neurotransmitters.
Endocrine
Signals from distant cells are called endocrine signals, and they originate from endocrine cells. In the body, many endocrine cells are located in endocrine glands, such as the thyroid gland, the hypothalamus and the pituitary gland. These types of signals usually produce a slower response but have a longer-lasting effect. The ligands released in endocrine signalling are called hormones, signalling molecules that are produced in one part of the body but affect other body regions some distance away.
Hormones travel the large distances between endocrine cells and their target cells via the bloodstream, which is a relatively slow way to move throughout the body. Because of their form of transport, hormones become diluted and are present in low or very low concentrations when they act on their target cells. This is different from paracrine signalling, in which local concentrations of ligands can be very high.
Excitable Cells
Another type of communication is via excitable cells. Cellular excitability refers to the ability to respond to changes in membrane potential caused by influx of ions. The signature trait of all excitable cells is the present of transitory electrical currents used to communicate with other cells. The two main types of excitable cells in the human body are the neurons of the nervous system and the autorhythmic cells of the cardiovascular system.
Neurons
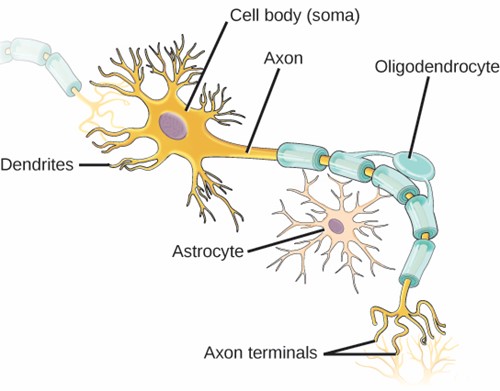
Nervous tissues are made of cells specialised to receive and transmit electrical impulses from specific areas of the body and to send them to specific locations in the body. The main cell of the nervous system is the neuron (Figure 5.22). The large structure with a central nucleus is the cell body of the neuron. Projections from the cell body are either dendrites specialised in receiving input or a single axon specialised in transmitting impulses.
Cardiac Autorhythmic Cells
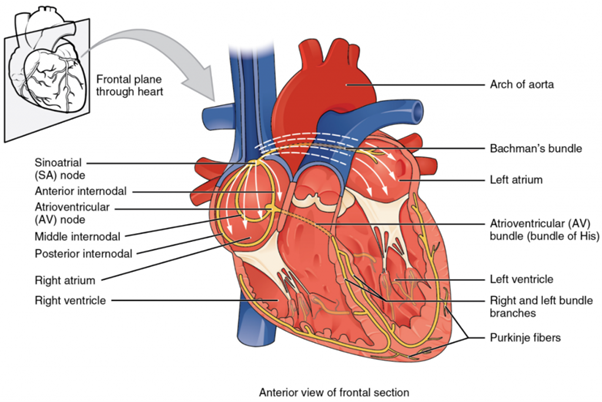
Some specialised cardiac cells (cells from cardiac muscle) have a slightly different way of communicating. These ‘pacemaker’ cells are autorhythmic, meaning they can start an electrical impulse themselves and then this signal is propagated throughout the heart and can instigate a muscle contraction used to move blood around and through the heart. If embryonic heart cells are separated into a Petri dish and kept alive, each can generate its own electrical impulse followed by a contraction. When two independently beating embryonic cardiac muscle cells are placed together, the cell with the higher inherent rate sets the pace, and the impulse spreads from the faster to the slower cell to trigger a contraction. As more cells are joined together, the fastest cell continues to assume control of the rate. A fully developed adult heart maintains the capability of generating its own electrical impulse, triggered by the fastest cells, as part of the cardiac (electrical) conduction system. The components of the cardiac (electrical) conduction system include the sinoatrial (SA) node (junction of upper wall of right atrium and opening of superior vena cava), the atrioventricular (AV) node (located near the coronary sinus on interatrial septum), the atrioventricular bundle (also known as the bundle of His; connects the atrial and ventricular chambers of the heart), the atrioventricular bundle branches (right and left branches located between atria and ventricles, and the Purkinje cells (inner ventricular walls of the heart in the subendocardium) (Figure 5.23).
Neurotransmitters
Some cell types require a specific molecule to bridge the (virtual) connection between cells to continue the communication impulse. There are two types of connections between electrically active cells such as neurons: chemical synapses and electrical synapses.
In a chemical synapse, a chemical signal—namely, a neurotransmitter—is released from one cell and it affects the other cell. This type of synapse is found at the neuromuscular junction (NMJ), which allows an electrical impulse to be converted to a chemical signal (neurotransmitter) that move across a space (synaptic cleft) to bind to a receptor to cause an effect. In the case of the NMJ, this effect would be muscle contraction.
In an electrical synapse, there is a direct connection between the two cells so that ions can pass directly from one cell to the next (eg intercalated discs in cardiac muscle which are gap junctions). If one cell is depolarised in an electrical synapse, the joined cell also depolarises because the ions pass between the cells. Chemical synapses involve the transmission of chemical information from one cell to the next.
Copyright Information: Sources from which this module has been adapted from can be found here.
Communication from one cell to another.
Communication within a cell, usually triggered in response to extracellular stimulation.
Cells that release chemical signals in the form of ligands.
A chemical messenger molecule that irreversibly binds a specific protein receptor to form a complex.
Cells that are affected by the chemical signals sent from signalling cells.
Biological macromolecule comprised of one or more amino acid chains.
Proteins either on the cell surface or inside a target cell that bind to a specific ligand and receives chemical signal.
An agonist is a molecule that activates a receptor to produce a biological response.
An antagonist is a molecule that blocks or inhibits the action of an agonist at a receptor, preventing a biological response.
An antagonist is a molecule that blocks or inhibits the action of an agonist at a receptor, preventing a biological response.
Increase in the number of cell-surface receptors to increase the cells response to stimulus.
Reduction in the number of cell-surface receptors to reduce or suppress the cells response to stimulus.
Chemotaxis is a biological process in which cells or organisms move in response to a chemical stimulus. The movement can be either toward (positive chemotaxis) or away from (negative chemotaxis) the source of the chemical signal.
Symbiosis refers to a close and often long-term interaction between two or more different biological species.
Communication between bacteria cells about cell population density that bacteria can then respond to by adjusting gene expression within the cell.
Signalling molecules secreted by bacteria to communicate with other bacteria of the same kind.
Property of a molecule to be repelled by, or not having an affinity for, water.
Short chain of amino acids linked together by peptide bonds. Peptides are smaller than proteins and can be thought of as small proteins or protein fragments. The distinction between peptides and proteins is somewhat arbitrary, but peptides are generally considered to contain fewer than 50 amino acids.
Type of enzyme that catalyses the transfer of a phosphate group from a high-energy molecule, such as adenosine triphosphate (ATP), to a specific target molecule, typically a protein. This process is known as phosphorylation.
A colony of bacteria attached to a biotic or abiotic surface enclosed within a matrix of extracellular polymeric substances produced by the colony.
Anything located or occurring outside a cell.
Free-swimming, unicellular bacteria.
Biological macromolecule comprised of one or more amino acid chains.
Functional length of DNA that provides the genetic information necessary to build a protein.
Polymeric refers to a structure that is made up of smaller, repeating units called monomers, which are covalently bonded together in a chain-like structure to form a larger, more complex molecule known as a polymer.
Organic polymers synthesised by microorganisms involved in colony aggregation as a biofilm and important for bacterial interactions with their environment.
Immune cell that surrounds, ingests and destroys foreign material.
Mucosa (plural: mucosae) refers to the moist tissue layer that lines certain parts of the inside of the body. It forms the innermost layer of certain organs and body cavities and serves various functions depending on its location.
Cystic fibrosis (CF) is a genetic disorder that affects mainly the lungs, but also the pancreas, liver, kidneys, and intestine. It is caused by mutations in the CFTR gene, which encodes a protein called the cystic fibrosis transmembrane conductance regulator.
Health condition or disease that persists over a long period of time.
Organic polymers synthesised by microorganisms involved in colony aggregation as a biofilm and important for bacterial interactions with their environment.
Set of observable characteristics, such as height, eye colour.
Membrane proteins found in the cell membranes of both prokaryotic and eukaryotic cells. They play a vital role in transporting various substrates, including ions, drugs, and metabolic products, out of the cell or into specific compartments within the cell.
Form of cell signalling in which a cell secretes a molecule, such as a hormone or growth factor, that binds to receptors on the same cell, leading to changes in the cell. Essentially, the cell targets itself, and the signalling molecule affects the cell that produced it.
Specialised intercellular connections that allow direct communication between the cytoplasm of adjacent cells. They enable various molecules, ions, and electrical impulses to pass directly from one cell to an adjacent cell, facilitating coordination and synchronization of activities among connected cells.
Type of cell-to-cell communication in which a cell produces a signal to induce changes in nearby cells, altering the behaviour or differentiation of those cells.
Type of intercellular communication that involves the secretion of hormones by endocrine glands into the bloodstream.
The signalling cell can also bind to the ligand being released enabling signalling to the same or a similar cell.
Specialised intercellular connections that allow direct communication between the cytoplasm of adjacent cells. They enable various molecules, ions, and electrical impulses to pass directly from one cell to an adjacent cell, facilitating coordination and synchronization of activities among connected cells.
Selectively permeable barrier that separates the interior of a cell from its external environment.
Positively or negatively charged atom or molecule.
Signalling molecule diffuse through extracellular matrix and target nearby cells.
Movement of molecules along the concentration gradient.
Three-dimensional network of proteins and molecules that surround cells to give structure and provide support.
Signalling molecule (hormone) enters the blood stream to signal a distant cell with a receptor specific to the signalling molecule.
System of glands and hormones that regulate many of the body's key functions by releasing hormones directly into the bloodstream. The endocrine system is vital for maintaining homeostasis and orchestrating complex physiological processes throughout the body.
Secretion of an endocrine organ that travels via the bloodstream or lymphatics to induce a response in target cells or tissues in another part of the body.
Type of cell-to-cell communication in which a cell produces a signal to induce changes in nearby cells, altering the behaviour or differentiation of those cells.
Specialised cells that have the ability to generate and propagate electrical signals known as action potentials.
Also known as a nerve cell, is the fundamental unit of the nervous system, responsible for transmitting information throughout the body. Neurons are electrically excitable cells that function to process and transmit information through electrical and chemical signals.
Ability of certain cells to generate a rhythm or regular pattern of electrical activity spontaneously.
Membrane-bound organelle found in most eukaryotic cells, often considered the control center of the cell because it houses the cell's genetic material, DNA (deoxyribonucleic acid).
Branched extensions of a neuron that act as the main information receivers for the cell.
Specialised extension of a neuron that conducts electrical impulses away from the neuron's cell body towards other neurons or effector cells, such as other neurons, muscles or glands.
Chemical signal is released from a neurotransmitter to communicate with target neuron.
Chemical substance that transmits signals across a synapse from one neuron (nerve cell) to another "target" neuron, muscle cell, or gland cell.
Protein molecule that contains a binding site for another specific molecule (called a ligand).
Direct connection between neurons allowing movement of ions from one cell to the next via gap junctions.
Depolarisation is a term used in biology to describe a change in a cell's membrane potential, specifically the voltage inside the cell when compared to voltage outside of the cell. Depolarisation occurs when the intracellular environment becomes more positively charged (less negative). It's a crucial process in the functioning of nerve and muscle cells.

