9 Common Cell Structures of the Prokaryotic and Eukaryotic Cells
In this section
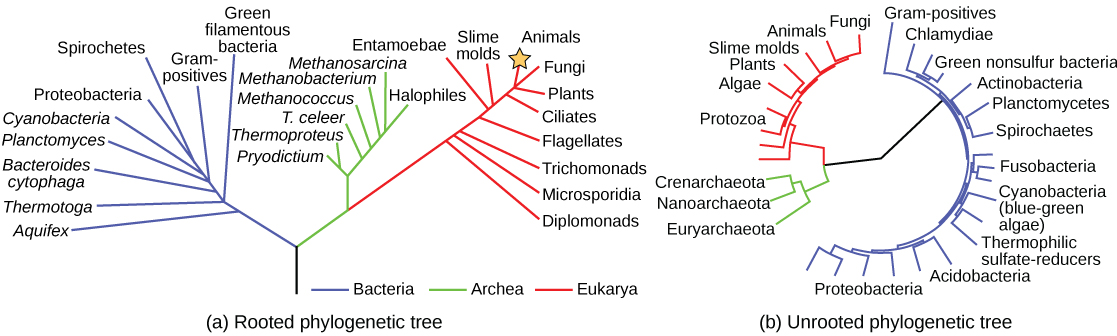
Despite sharing certain characteristics, cells may vary significantly. The two main types of cells are prokaryotic cells (lacking a nucleus) and eukaryotic cells (containing a well-organised, membrane-bound nucleus). Each type of cell exhibits a remarkable variety in structure, function and metabolic activity (Figure 3.1). There are some structures found in prokaryotic cells that are similar to those found in some eukaryotic cells; others are unique to prokaryotes. A good example of this is how and where genetic material is located in the different cell types; prokaryotic cells generally have a single, circular chromosome located in a nucleoid whereas eukaryotic cells have a defined nucleus, surrounded by a complex nuclear membrane containing multiple, rod-shaped chromosomes. Although there are some exceptions, eukaryotic cells tend to be larger than prokaryotic cells and the larger size of eukaryotic cells dictates the need to compartmentalise various chemical processes within different areas of the cell, using complex membrane-bound organelles.
All plant cells and animal cells are eukaryotic. Some microorganisms are prokaryotic cells, whereas others are eukaryotic cells. Prokaryotic microorganisms are classified within the domains Archaea and Bacteria, whereas eukaryotic organisms are classified within the domain Eukarya (Figure 3.1). There are also a few other intermediate types of microorganisms and so the division between prokaryote and eukaryote can be blurred as new microbial species are discovered and characterised.
This chapter will focus on the distinguishing structures found in prokaryotic and eukaryotic cells.
Genetic Information Directs Cell Function
A cell’s complete complement of DNA is called its genome. In prokaryotes (“before nucleus”), the genome is composed of a single, double-stranded DNA molecule in the form of a loop or circle. The region in the cell containing this genetic material is called a nucleoid. Eukaryotic (“true nucleus”) cells typically have their DNA organised into multiple linear chromosomes and these are found in a discrete area known as a nucleus. The DNA within the nucleus is highly organised and condensed to fit inside the nucleus, which is accomplished by wrapping the DNA around proteins called histones.
DNA serves two essential functions that deal with cellular information. First, DNA is the genetic material responsible for inheritance and is passed from parent to offspring for all life on earth. To preserve the integrity of this genetic information, DNA must be replicated with great accuracy, with minimal errors which would introduce changes to the DNA sequence. A genome contains the full complement of DNA within a cell and is organised into smaller, discrete units called genes that are arranged on chromosomes and plasmids. The second function of DNA is to direct and regulate the construction of the proteins necessary to a cell for growth and reproduction in a particular cellular environment.
A gene is composed of DNA that is “read” or transcribed to produce an RNA molecule during the process of transcription. One major type of RNA molecule, called messenger RNA (mRNA), provides the information for the ribosome to catalyse protein synthesis in a process called translation. The processes of transcription and translation are collectively referred to as gene expression mostly resutling in the synthesis of a specific protein with a sequence of amino acids that is encoded in the gene. The flow of genetic information from DNA to RNA to protein is described by the central dogma (Figure 3.2).
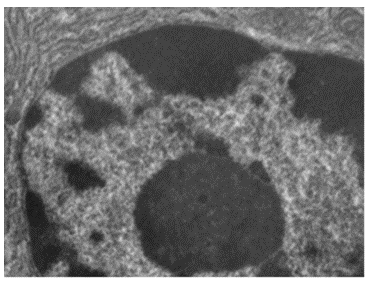
A cell’s genotype is the full collection of genes it contains, whereas its phenotype is the set of observable characteristics that result from those genes, such as height, eye colour and blood type. The phenotype is the product of the array of proteins being produced by the cell at a given time, which is influenced by the cell’s genotype as well as interactions with the cell’s environment. Genes code for proteins that have functions in the cell. Production of a specific protein encoded by an individual gene often results in a distinct phenotype for the cell compared with the phenotype without that protein. For this reason, it is also common to refer to the genotype of an individual gene and its phenotype. Although a cell’s genotype remains constant, not all genes are used to direct the production of their proteins simultaneously. Cells carefully regulate expression of their genes, only using genes to make specific proteins when those proteins are needed (Figure 3.3).
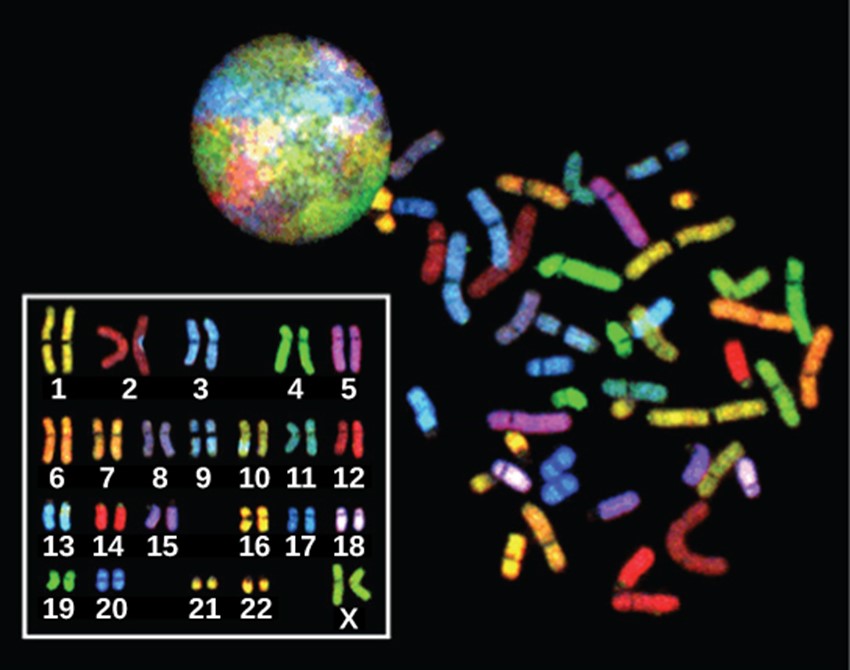
In eukaryotes, the genome comprises several double-stranded, linear DNA molecules bound with proteins to form complexes called chromosomes. Each species of eukaryote has a characteristic number of chromosomes in the nuclei of its cells. Human body cells (somatic cells) have 46 chromosomes (Figure 3.4). A somatic cell contains two matched sets of chromosomes, a configuration known as diploid. The letter n is used to represent a single set of chromosomes; therefore a diploid organism is designated 2n. Human cells that contain one set of 23 chromosomes are called gametes, or sex cells; these eggs and sperm are designated n, or haploid.
The matched pairs of chromosomes in a diploid organism are called homologous chromosomes. Homologous chromosomes are the same length and have specific nucleotide segments called genes in exactly the same location, or locus. Genes, the functional units of chromosomes, determine specific characteristics by coding for specific proteins. Traits are the different forms of a characteristic, for example, the shape of earlobes is a characteristic with traits of free or attached.
Each copy of the homologous pair of chromosomes originates from a different parent; therefore, the copies of each of the genes themselves may not be identical. The variation of individuals within a species is caused by the specific combination of the genes inherited from both parents. For example, there are three possible gene sequences on the human chromosome that codes for blood type: sequence A, sequence B, and sequence O. Because all diploid human cells have two copies of the chromosome that determines blood type, the blood type (the trait) is determined by which two versions of the marker gene are inherited. It is possible to have two copies of the same gene sequence, one on each homologous chromosome (for example, AA, BB, or OO), or two different sequences, such as AB.
Minor variations in traits such as those for blood type, eye colour, and height contribute to the natural variation found within a species. The sex chromosomes of humans, X and Y, are the single exception to the rule of homologous chromosomes; other than a small amount of homology that is necessary to reliably produce gametes, the genes found on the X and Y chromosomes are not the same.
Nucleic Acids – DNA and RNA
Nucleic acids are key macromolecules in the continuity of life. They carry the genetic blueprint of a cell and carry instructions for the functioning of the cell. The two main types of nucleic acids are deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). DNA is the genetic material found in all living organisms, ranging from single-celled bacteria to multicellular mammals.
The other type of nucleic acid, RNA, is mostly involved in protein synthesis. The DNA molecules never leave the nucleus, but instead use an RNA intermediary to communicate with the rest of the cell. Other types of RNA are also involved in protein synthesis and its regulation.
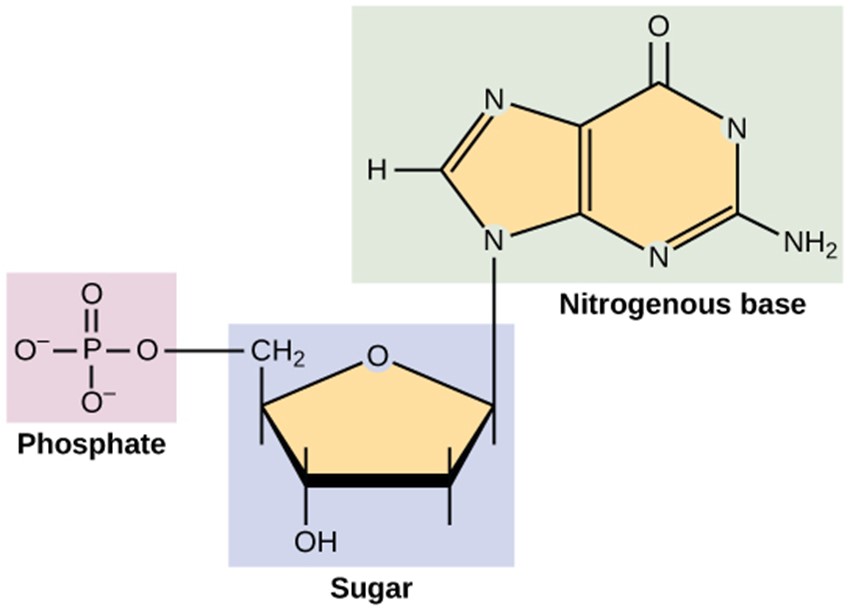
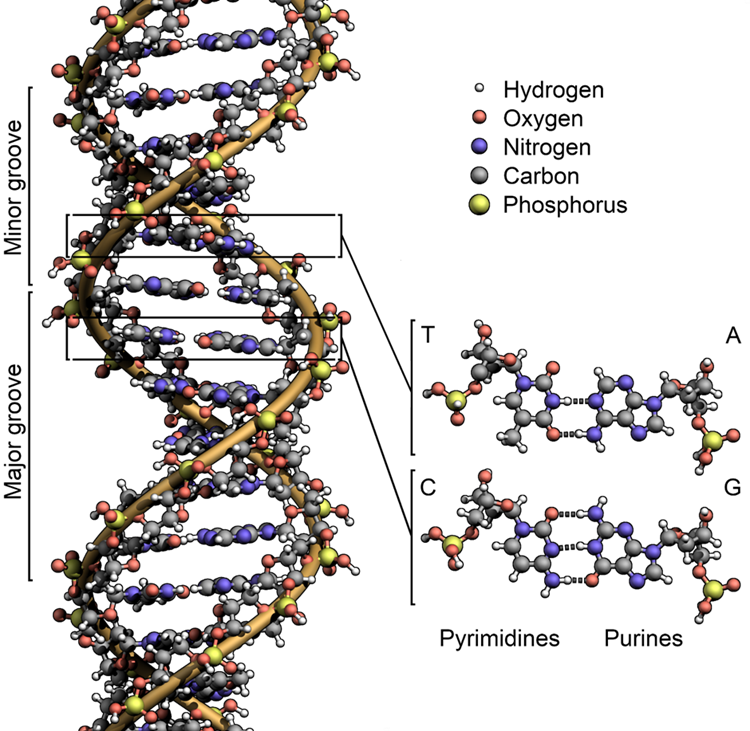
DNA and RNA are made up of monomers known as nucleotides. The nucleotides combine with each other to form a polynucleotide, DNA or RNA. Each nucleotide is made up of three components: a nitrogenous base, a pentose (five-carbon) sugar, and a phosphate group (Figure 3.5). Each nitrogenous base in a nucleotide is attached to a sugar molecule, which is attached to a phosphate group. The alternating sugar and phosphate groups lie on the outside of each strand, forming the backbone of the DNA. The nitrogenous bases adenine (A), cytosine (C), guanine (G) and thymine (T) are stacked in the interior, like the steps of a staircase, and these bases form a pair; the pairs are bound to each other by hydrogen bonds. The bases pair in such a way that the distance between the backbones of the two strands is the same all along the molecule (Figure 3.6).
DNA has a double-helical structure (Figure 3.7). It is composed of two strands, or polymers, of nucleotides. The strands are formed with bonds between phosphate and sugar groups of adjacent nucleotides. The strands are bonded to each other at their bases with hydrogen bonds, and the strands coil about each other along their length, hence the “double helix” description, which means a double spiral.
Cells may also contain extrachromosomal DNA, or DNA that is not part of the chromosome. This extrachromosomal DNA is found in plasmids, which are small, circular, double-stranded DNA molecules. Cells that have plasmids often have hundreds of them within a single cell. Plasmids are more commonly found in bacteria; however, plasmids have been found in archaea and eukaryotic organisms. Plasmids often carry genes that confer advantageous traits such as antibiotic resistance; thus, they are important to the survival of the organism.
The other type of nucleic acid, RNA, is mostly involved in protein synthesis. Just like in DNA, RNA is made of monomers called nucleotides. Each nucleotide is made up of three components: a nitrogenous base, a pentose (five-carbon) sugar called ribose, and a phosphate group. Each nitrogenous base in a nucleotide is attached to a sugar molecule, which is attached to one or more phosphate groups (Figure 3.8).
In RNA, the nitrogenous bases vary slightly from those of DNA. Adenine (A), guanine (G), and cytosine (C) are present, but instead of thymine (T), a pyrimidine called uracil (U) pairs with adenine. RNA is a single stranded molecule, compared to the double helix of DNA.
The DNA molecules never leave the nucleus but instead use an intermediary to communicate with the rest of the cell. This intermediary is the messenger RNA (mRNA). When proteins need to be made, the mRNA enters the nucleus and attaches itself to one of the DNA strands. Being complementary, the sequence of nitrogen bases of the RNA is opposite that of the DNA. This is called transcription. For example, if the DNA strand reads TCCAAGTC, then the mRNA strand would read AGGUUCAG. The mRNA then carries the code out of the nucleus to organelles called ribosomes for the assembly of proteins.
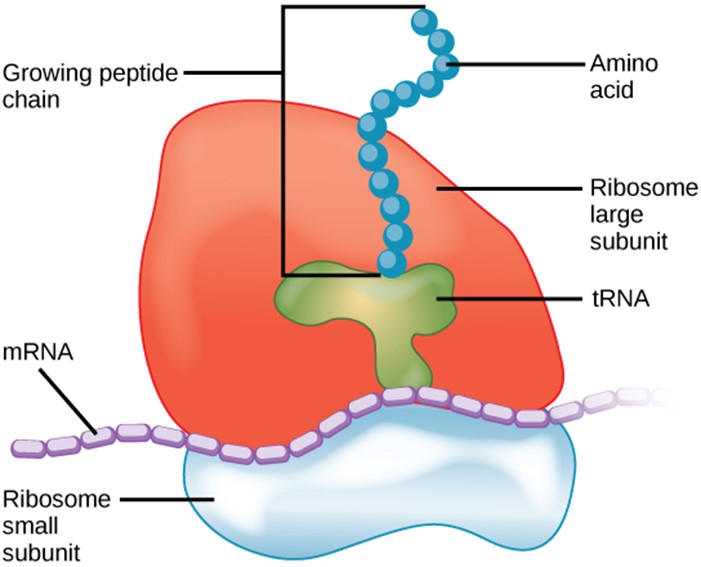
Once the mRNA has reached the ribosomes, they do not read the instructions directly. Instead, another type of RNA called transfer RNA (tRNA) needs to translate the information from the mRNA into a usable form. The tRNA attaches to the mRNA, but with the opposite base pairings. It then reads the sequence in sets of three bases called codons. Each possible three letter arrangement of A,C,U,G (eg AAA, AAU, GGC, etc) is a specific instruction, and the correspondence of these instructions and the amino acids is known as the “genetic code.” Though exceptions to or variations on the code exist, the standard genetic code holds true in most organisms.
The ribosome acts like a giant clamp, holding all of the players in position, and facilitating both the pairing of bases between the messenger and transfer RNAs, and the chemical bonding between the amino acids. The ribosome has special subunits known as ribosomal RNAs (rRNA) because they function in the ribosome. These subunits do not carry instructions for making a specific proteins (i.e., they are not messenger RNAs) but instead are an integral part of the ribosome machinery that is used to make proteins from mRNAs. The making of proteins by reading instructions in mRNA is generally known as ”translation.”
Cell Walls Protecting Cellular Components
Structures that enclose the cytoplasm and internal structures of the cell are known collectively as the cell envelope. Cells of fungi, algae, plants, and even some protists have cell walls. Depending upon the type of eukaryotic cell, cell walls can be made of a wide range of materials, including cellulose (fungi and plants); biogenic silica, calcium carbonate, agar, and carrageenan (protists and algae); or chitin (fungi). In general, all cell walls provide structural stability for the cell and protection from environmental stresses such as desiccation, changes in osmotic pressure, and traumatic injury.
In prokaryotic cells, the structures of the cell envelope vary depending on the type of cell and organism. Most (but not all) prokaryotic cells have a cell wall, but the makeup of this cell wall varies.
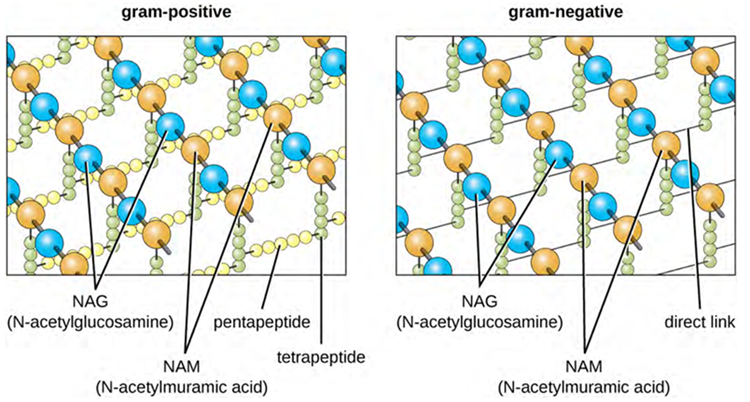
The major component of bacterial cell walls is called peptidoglycan (or murein); it is only found in bacteria. Structurally, peptidoglycan resembles a layer of meshwork or fabric (Figure 3.9). Each layer is composed of long chains of alternating molecules of N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). The structure of the long chains has significant two-dimensional tensile strength due to the formation of peptide bridges that connect NAG and NAM within each peptidoglycan layer. In gram-negative bacteria, tetrapeptide chains extending from each NAM unit are directly cross-linked, whereas in gram-positive bacteria, these tetrapeptide chains are linked by pentaglycine cross-bridges. Peptidoglycan subunits are made inside of the bacterial cell and then exported and assembled in layers, giving the cell its shape.
Since peptidoglycan is unique to bacteria, many antibiotic drugs are designed to interfere with peptidoglycan synthesis, weakening the cell wall and making bacterial cells more susceptible to the effects of osmotic pressure. In addition, certain cells of the human immune system are able to “recognise” bacterial pathogens by detecting peptidoglycan on the surface of a bacterial cell; these cells then engulf and destroy the bacterial cell, using enzymes such as lysozyme, which breaks down and digests the peptidoglycan in their cell walls.
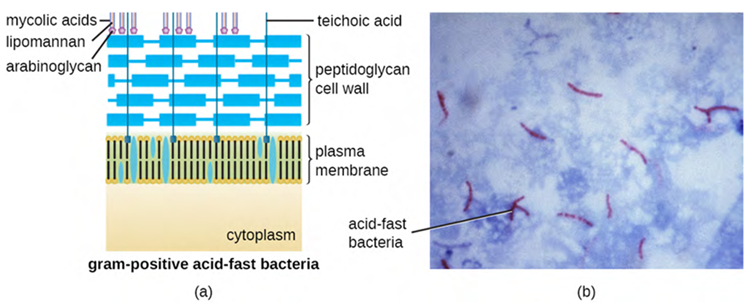
The Gram staining protocol (used in staining microscopic specimens) is used to differentiate two common types of cell wall structures (Figure 3.10). Gram-positive cells have a cell wall consisting of many layers of peptidoglycan totalling 30–100 nm in thickness. These peptidoglycan layers are commonly embedded with teichoic acids (TAs), carbohydrate chains that extend through and beyond the peptidoglycan layer. TA is thought to stabilise peptidoglycan by increasing its rigidity. TA also plays a role in the ability of pathogenic gram-positive bacteria such as Streptococcus spp. to bind to certain proteins on the surface of host cells, enhancing their ability to cause infection. In addition to peptidoglycan and TAs, bacteria of the family Mycobacteriaceae have an external layer of waxy mycolic acids in their cell wall (affecting stain infiltration) and these bacteria are referred to as acid-fast, since acid-fast stains must be used to penetrate the mycolic acid layer for purposes of microscopy (Figure 3.11).
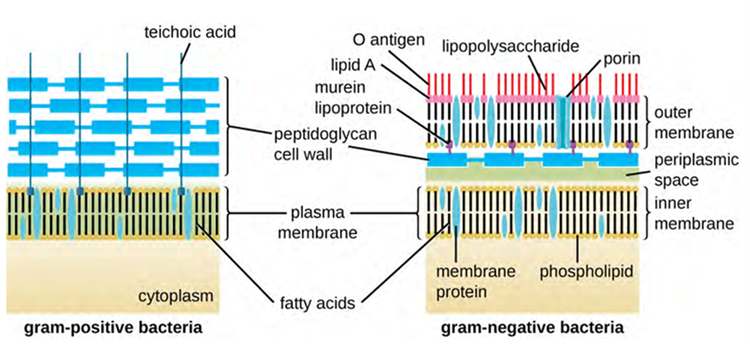
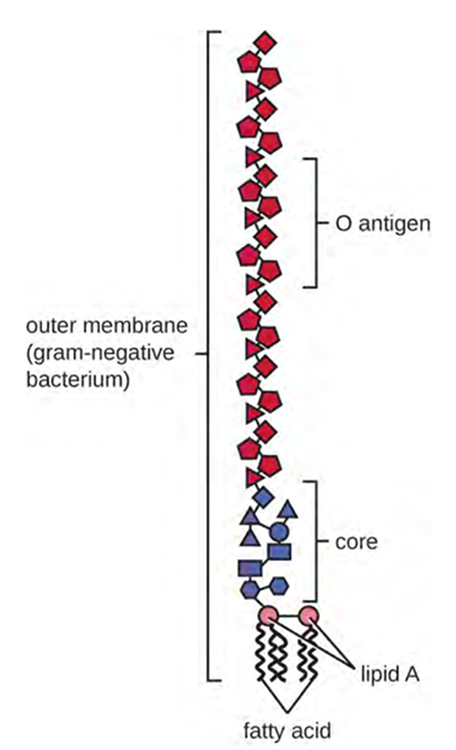
Gram-negative cells have a much thinner layer of peptidoglycan (no more than about 4 nm thick) than gram-positive cells, and the overall structure of their cell envelope is more complex. In gram-negative cells, a gel-like matrix occupies the periplasmic space between the cell wall and the plasma membrane, and there is a second lipid bilayer called the outer membrane, which is external to the peptidoglycan layer (Figure 3.12). This outer membrane is attached to the peptidoglycan by murein lipoprotein. The outer leaflet of the outer membrane contains the molecule lipopolysaccharide (LPS), which functions as an endotoxin in infections involving gram-negative bacteria, contributing to symptoms such as fever, haemorrhaging and septic shock. Each LPS molecule is composed of Lipid A, a core polysaccharide, and an O side chain that is composed of sugar-like molecules that comprise the external face of the LPS (Figure 3.13). The composition of the O side chain varies between different species and strains of bacteria. Parts of the O side chain called antigens can be detected using serological or immunological tests to identify specific pathogenic strains like Escherichia coli O157:H7, a pathogenic strain of bacteria that causes bloody diarrhea and kidney failure.
Archaeal cell wall structure differs from that of bacteria in several significant ways. First, archaeal cell walls do not contain peptidoglycan; instead, they contain a similar polymer called pseudopeptidoglycan (pseudomurein) in which NAM is replaced with a different subunit. Other archaea may have a layer of glycoproteins or polysaccharides that serves as the cell wall instead of pseudopeptidoglycan. Last, as is the case with some bacterial species, there are a few archaea that appear to lack cell walls entirely.
Depending upon the type of eukaryotic cell (fungi, algae, plants etc), cell walls can be made of a wide range of materials, including cellulose (fungi and plants); biogenic silica, calcium carbonate, agar, and carrageenan (protists and algae); or chitin (fungi). With some fungal infections, specific components of the cell wall are targeted by antibiotics.
Cytoskeleton
Both prokaryotic cells and eukaryotic cells have an internal cytoskeleton. The bacterial cytoskeleton helps sense the environment and ensure communication between cells. These communications are aided by conjugation tubes and nanotubes and even by membrane vesicles and can also confer increased virulence when certain groups of bacteria form a biofilm or are located in the same vicinity.
Much more is known about the cytoskeleton of eukaryotic cells and these are made of microfilaments, intermediate filaments, and microtubules. This matrix of fibres and tubes provides structural support as well as a network over which materials can be transported within the cell and on which organelles can be anchored (Figure 3.13). For example, the process of exocytosis involves the movement of a vesicle via the cytoskeletal network to the plasma membrane, where it can release its contents.
The eukaryotic cytoskeleton consists of a network of microfilaments, intermediate filaments and microtubules located in the cytoplasm.
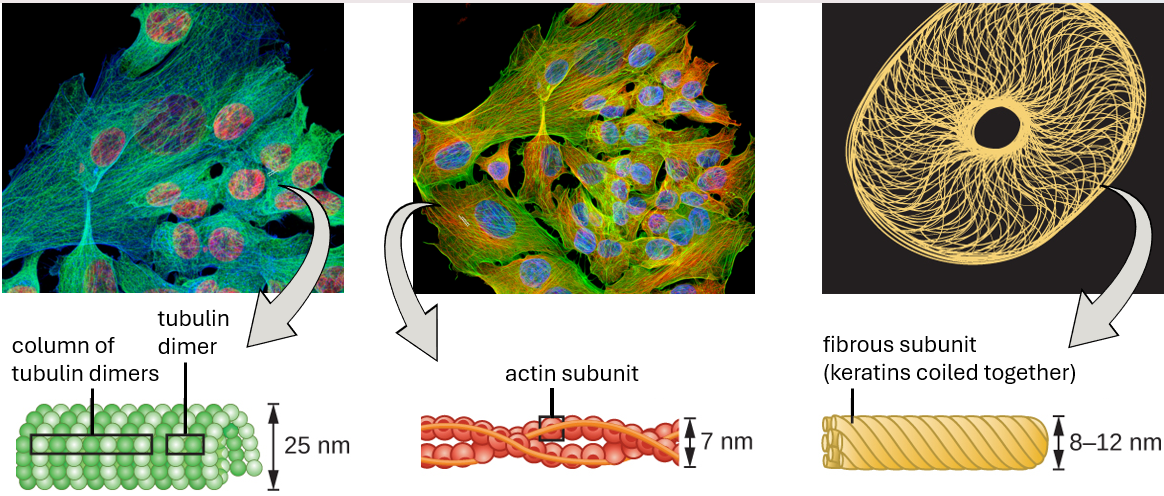
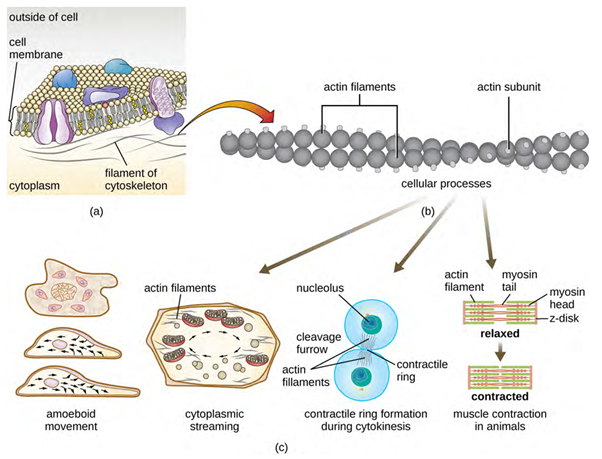
Microfilaments are composed of two intertwined strands of actin, each composed of actin monomers forming filamentous cables 6 nm in diameter (Figure 3.14). The actin filaments work together with motor proteins, like myosin, to effect muscle contraction in animals or the amoeboid movement of some eukaryotic microbes. In ameboid organisms, actin can be found in two forms: a stiffer, polymerised, gel form and a more fluid, unpolymerised soluble form. Actin in the gel form creates stability in the ectoplasm, the gel-like area of cytoplasm just inside the plasma membrane of ameboid protozoans.
Adenosine triphosphate (ATP) powers actin to assemble its filamentous form, which serves as a track for the movement of a motor protein we call myosin. This enables actin to engage in cellular events requiring motion, such as cell division in eukaryotic cells and cytoplasmic streaming, which is the cell cytoplasm’s circular movement in plant cells. Actin and myosin are plentiful in muscle cells. When your actin and myosin filaments slide past each other, your muscles contract.
Temporary extensions of the cytoplasmic membrane called pseudopodia (meaning “false feet”) are produced through the forward flow of soluble actin filaments into the pseudopodia, followed by the gel-sol cycling of the actin filaments, resulting in cell motility. Once the cytoplasm extends outward, forming a pseudopodium, the remaining cytoplasm flows up to join the leading edge, thereby creating forward locomotion. Beyond amoeboid movement, microfilaments are also involved in a variety of other processes in eukaryotic cells, including cytoplasmic streaming (the movement or circulation of cytoplasm within the cell), cleavage furrow formation during cell division, and muscle movement in animals (Figure 3.14). These functions are the result of the dynamic nature of microfilaments, which can polymerise and depolymerise relatively easily in response to cellular signals, and their interactions with molecular motors in different types of eukaryotic cells.
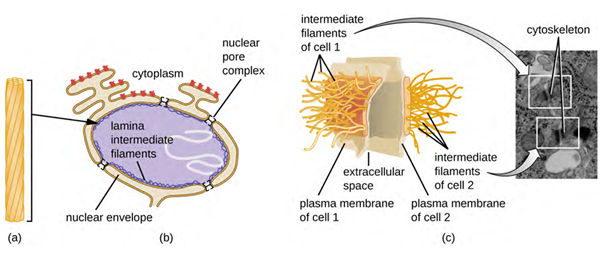
Intermediate filaments (Figure 3.15) are a diverse group of cytoskeletal filaments that act as cables within the cell. They are termed “intermediate” because their 10-nm diameter is thicker than that of actin but thinner than that of microtubules. They are composed of several strands of polymerised subunits that, in turn, are made up of a wide variety of monomers. Intermediate filaments tend to be more permanent in the cell and maintain the position of the nucleus. They also form the nuclear lamina (lining or layer) just inside the nuclear envelope. Additionally, intermediate filaments play a role in anchoring cells together in animal tissues. The intermediate filament protein desmin is found in desmosomes, the protein structures that join muscle cells together and help them resist external physical forces. The intermediate filament protein keratin is a structural protein found in hair, skin, and nails.
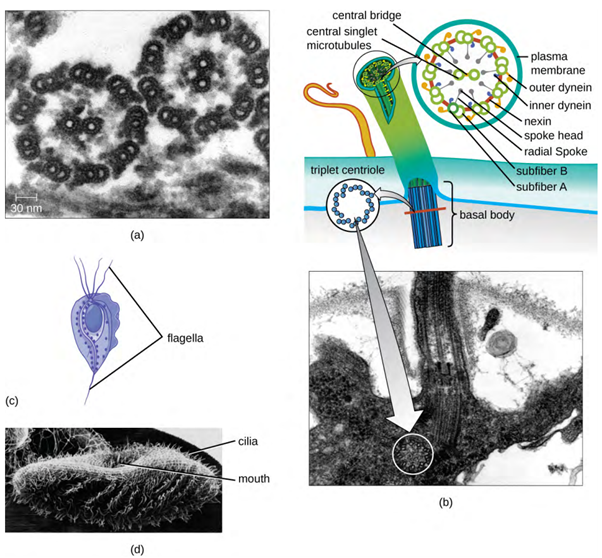
Microtubules (Figure 3.16) are a third type of cytoskeletal fibre composed of tubulin dimers (α tubulin and β tubulin). These form hollow tubes 23 nm in diameter that are used as girders within the cytoskeleton. Like microfilaments, microtubules are dynamic and have the ability to rapidly assemble and disassemble. Microtubules also work with motor proteins (such as dynein and kinesin) to move organelles and vesicles around within the cytoplasm. Additionally, microtubules are the main components of eukaryotic flagella and cilia, composing both the filament and the basal body components (Figure 3.15).
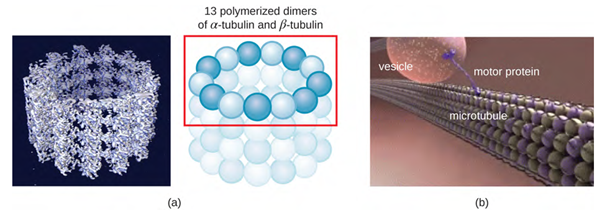
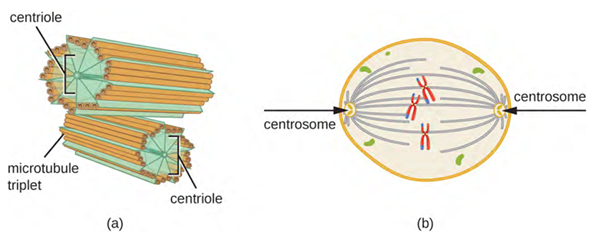
In addition, microtubules are involved in cell division, forming the mitotic spindle that serves to separate chromosomes during mitosis and meiosis. The mitotic spindle is produced by two centrosomes, which are essentially microtubule-organising centres, at opposite ends of the cell. Each centrosome is composed of a pair of centrioles positioned at right angles to each other, and each centriole is an array of nine parallel microtubules arranged in triplets (Figure 3.17).
Take a look at this comparison chart on eukaryotic cells vs prokaryotic cells.
Plasma Membranes
All cells (prokaryotic and eukaryotic) have a plasma membrane (also called cytoplasmic membrane or cell membrane) that exhibits selective permeability, allowing some molecules to enter or leave the cell while restricting the passage of others.
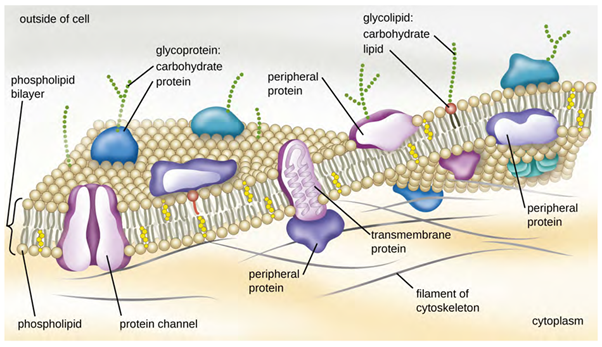
The structure of the plasma membrane is often described in terms of the fluid mosaic model, which refers to the ability of membrane components to move fluidly within the plane of the membrane, as well as the mosaic-like composition of the components, which include a diverse array of lipid and protein components (Figure 3.18). The plasma membrane structure of most bacterial and eukaryotic cell types is a bilayer composed mainly of phospholipids formed with ester linkages and proteins. These phospholipids and proteins have the ability to move laterally within the plane of the membranes as well as between the two phospholipid layers.
Archaeal membranes are fundamentally different from bacterial and eukaryotic membranes in a few significant ways. First, archaeal membrane phospholipids are formed with ether linkages, in contrast to the ester linkages found in bacterial or eukaryotic cell membranes. Second, archaeal phospholipids have branched chains, whereas those of bacterial and eukaryotic cells are straight chained. Finally, although some archaeal membranes can be formed of bilayers like those found in bacteria and eukaryotes, other archaeal plasma membranes are lipid monolayers.
Proteins on the cell’s surface are important for a variety of functions, including cell-to-cell communication, and sensing environmental conditions and pathogenic virulence factors. Membrane proteins and phospholipids may have carbohydrates (sugars) associated with them and are called glycoproteins or glycolipids, respectively. These glycoprotein and glycolipid complexes extend out from the surface of the cell, allowing the cell to interact with the external environment (Figure 3.18). Glycoproteins and glycolipids in the plasma membrane can vary considerably in chemical composition among archaea, bacteria, and eukaryotes, allowing scientists to use them to characterise unique species.
Plasma membranes from different cell types also contain unique phospholipids, which contain fatty acids. These, phospholipid-derived fatty acid analysis (PLFA) profiles can be used to identify unique types of cells based on differences in fatty acids. Archaea, bacteria, and eukaryotes each have a unique PFLA profile.
Membrane Transport Mechanisms
One of the most important functions of the plasma membrane (a semipermeable membrane) is to control the transport of molecules into and out of the cell. Internal conditions must be maintained within a certain range despite any changes in the external environment. The transport of substances across the plasma membrane allows cells to maintain the intracellular environment.
Cells use various modes of transport across the plasma membrane. Some small molecules, like carbon dioxide or water, may cross the membrane bilayer directly by simple diffusion. This is when molecules move from an area of higher concentration to an area of lower concentration and move with the concentration gradient – simple diffusion is also known as passive transport and doesn’t require energy to occur (Figure 3.19).

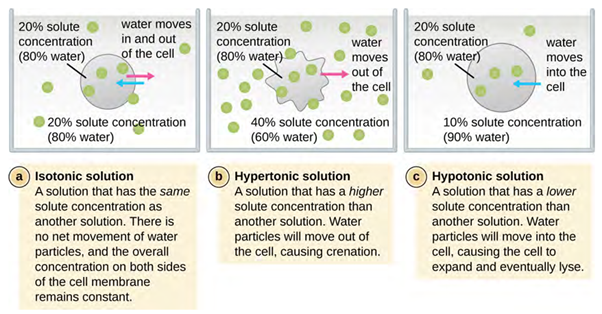
Osmosis (movement of water) is a special type of passive transport process which protects the cell from changes in osmotic pressure (Figure 3.20). Osmotic pressure occurs because of differences in the concentration of solutes on opposing sides of a semipermeable membrane. Water can pass through a semipermeable membrane, but solutes (dissolved molecules like salts, sugars and other compounds) cannot. When the concentration of solutes is greater on one side of the membrane, water diffuses across the membrane from the side with the lower concentration of solutes (more water) to the side with the higher concentration of solutes (less water) until the concentrations on both sides become equal. This diffusion/movement of water is called osmosis and it can cause extreme osmotic pressure on a cell when its external environment changes relative to the concentration inside the cell.
The degree to which a particular cell can withstand changes in osmotic pressure is called tonicity (the tone of the cell and how it changes shape). Cells that have a cell wall are better able to withstand subtle changes in osmotic pressure and maintain their shape. In hypertonic environments, cells that lack a cell wall can become dehydrated, causing crenation, or shrivelling of the cell; the plasma membrane contracts and appears scalloped or notched (Figure 3.20). By contrast, cells that possess a cell wall undergo plasmolysis rather than crenation. In plasmolysis, the plasma membrane contracts and detaches from the cell wall and there is a decrease in interior volume, but the cell wall remains intact, thus allowing the cell to maintain some shape and integrity for a period of time (Figure 3.15). Likewise, cells that lack a cell wall are more prone to lysis in hypotonic environments. The presence of a cell wall allows the cell to maintain its shape and integrity for a longer time before lysing (Figure 3.20). The most common examples of the effect of hypertonic and hypotonic are using plant cell walls (Figure 3.21) and also red blood cells (no cell wall) (Figure 3.22) or bacterial cells.
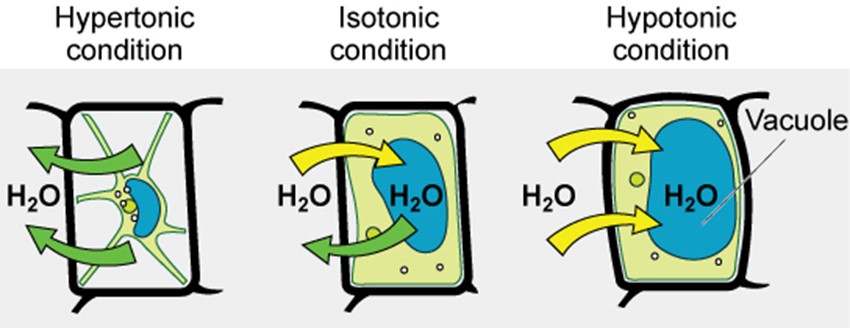
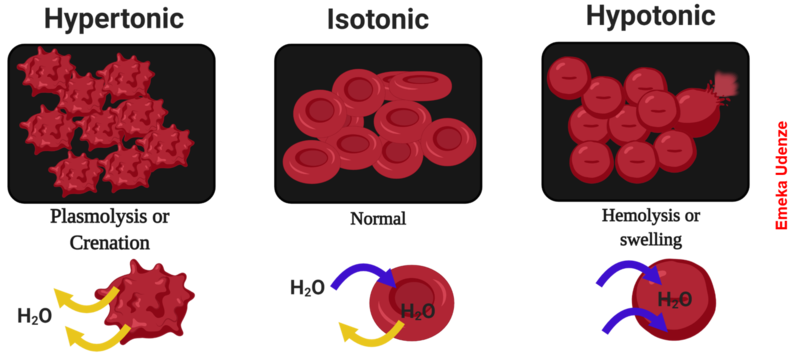
In prokaryotic cells, the cell wall provides some protection against changes in osmotic pressure, allowing it to maintain its shape longer. The cell membrane is typically attached to the cell wall in an isotonic medium (left). In a hypertonic medium, the cell membrane detaches from the cell wall and contracts (plasmolysis) as water leaves the cell. In a hypotonic medium (right), the cell wall prevents the cell membrane from expanding to the point of bursting, although lysis will eventually occur if too much water is absorbed.
Other molecules other than water, such as charged molecules and large molecules, need the help of carriers or channels in the membrane. These carriers or channels serve to ferry molecules across the membrane, a process known as facilitated diffusion and doesn’t require energy to work (Figure 3.23).
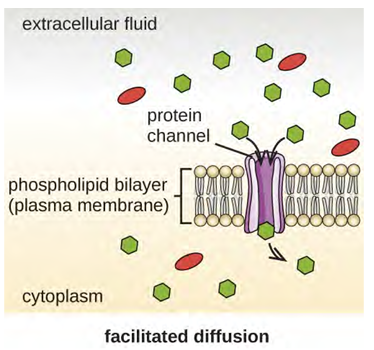
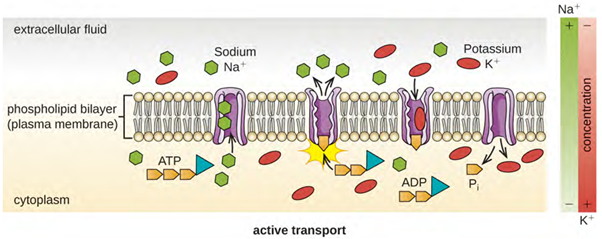
Active transport, however, does need energy to move molecules across the cell membrane against a concentration gradient (Figure 3.24). A major difference between passive and active transport is that active transport requires adenosine triphosphate (ATP) or other forms of energy to move molecules “uphill.” Therefore, active transport structures are often called “pumps.”
Group translocation also transports substances into cells. In this case, as a molecule moves into a cell against its concentration gradient, it is chemically modified so that it does not require transport against an unfavourable concentration gradient. A common example of this is the bacterial phosphotransferase system, a series of carriers that phosphorylates (ie adds phosphate ions to) glucose or other sugars upon entry into cells. Since the phosphorylation of sugars is required during the early stages of sugar metabolism, the phosphotransferase system is considered to be an energy neutral system.
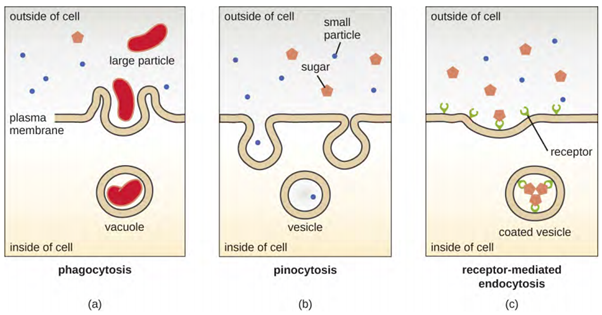
The processes of simple diffusion, facilitated diffusion, and active transport are used in both eukaryotic and prokaryotic cells. However, eukaryotic cells also have the unique ability to perform diverse types of endocytosis, the uptake of matter through plasma membrane invagination and vacuole/vesicle formation (Figure 3.25). A type of endocytosis involving the engulfment of large particles through membrane invagination is called phagocytosis, which means “cell eating.” In phagocytosis, particles (or other cells) are enclosed in a pocket within the membrane, which then pinches off from the membrane to form a vacuole that surrounds the particle. Another type of endocytosis is called pinocytosis, which means “cell drinking.” In pinocytosis, small, dissolved materials and liquids are taken into the cell through small vesicles. Saprophytic fungi, for example, obtain their nutrients from dead and decaying matter through pinocytosis.
Receptor-mediated endocytosis is a type of endocytosis that is initiated by specific molecules called ligands when they bind to cell surface receptors on the membrane. Receptor-mediated endocytosis is the mechanism that peptide and amine-derived hormones use to enter cells and is also used by various viruses and bacteria for entry into host cells.
The process by which secretory vesicles release their contents to the cell’s exterior is called exocytosis. Vesicles move toward the plasma membrane and then meld with the membrane, ejecting their contents out of the cell. Exocytosis is used by cells to remove waste products and may also be used to release chemical signals that can be taken up by other cells.
Ribosomes
All cellular life synthesises proteins and organisms in the bacteria, archaea and eukaryotic domains (Figure 3.26) – beginning of Section) of life possess ribosomes, structures responsible for protein synthesis. However, ribosomes in each of the three domains are structurally different. Ribosomes, themselves, are constructed from proteins, along with ribosomal RNA (rRNA). Prokaryotic ribosomes are found in the cytoplasm and are called 70S ribosomes because they have a size of 70S with two subunits, whereas eukaryotic cytoplasmic ribosomes have a size of 80S, again with two subunits. In terms of size and composition, this makes them distinct from the ribosomes of prokaryotic cells. The S stands for Svedberg unit, a measure of sedimentation in an ultracentrifuge, which is based on size, shape, and surface qualities of the structure being analysed. Although they are the same size, bacterial and archaeal ribosomes have different proteins and rRNA molecules, and the archaeal versions are more like their eukaryotic counterparts than to those found in bacteria.
The two types of non-organelle-associated eukaryotic ribosomes are defined by their location in the cell: free ribosomes and membrane-bound ribosomes. Free ribosomes are found in the cytoplasm and serve to synthesise water-soluble proteins; membrane-bound ribosomes are found attached to the rough endoplasmic reticulum and make proteins for insertion into the cell membrane or proteins destined for export from the cell.
The differences between eukaryotic and prokaryotic ribosomes are clinically relevant because certain antibiotic drugs are designed to target one or the other. For example, cycloheximide targets eukaryotic action, whereas chloramphenicol targets prokaryotic ribosomes. Since human cells are eukaryotic, they generally are not harmed by antibiotics that destroy the prokaryotic ribosomes in bacteria. However, sometimes negative side effects may occur because mitochondria in human cells contain prokaryotic ribosomes.
Cell Movement
Cells, both prokaryotic and eukaryotic, are not static entities. Microorganisms such as bacteria need to move from host to host or towards nutrients, eukaryotic cells such as sperm need to move to specific locations in the body or protozoa like Giardia need to move in their environment. The different cellular motility methods are discussed below.
Prokaryotic Cells
Bacterial movement typically involves the use of flagella, although there are a few other possibilities as well (such as the use of type IV pili for twitching motility), however the most common type of bacterial movement is swimming, which is accomplished with the use of a flagellum (singular) or flagella (plural).
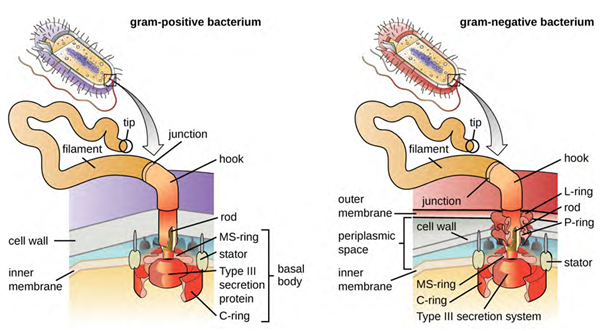
Flagella are structures used by cells to move in aqueous environments. Bacterial flagella act like propellers. They are stiff spiral filaments composed of flagellin protein subunits that extend outward from the cell and spin in solution. The basal body is the motor for the flagellum and is embedded in the plasma membrane (Figure 3.27). A hook region connects the basal body to the filament. Gram-positive and gram-negative bacteria have different basal body configurations due to the differences in cell wall structure.
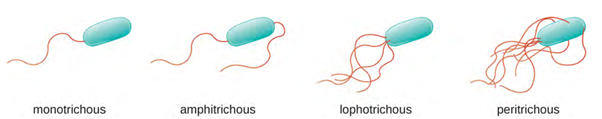
Different types of motile bacteria exhibit different arrangements of flagella (Figure 3.28). A bacterium with a singular flagellum, typically located at one end of the cell (polar), is said to have a monotrichous flagellum. An example of a monotrichously flagellated bacterial pathogen is Vibrio cholerae, the gram-negative bacterium that causes cholera. Cells with amphitrichous flagella have a flagellum or tufts of flagella at each end. An example is Spirillum minor, the cause of spirillary (Asian) rat-bite fever (also known as sodoku). Cells with lophotrichous flagella have a tuft at one end of the cell. The gram-negative bacillus Pseudomonas aeruginosa, an opportunistic pathogen known for causing many infections, including “swimmer’s ear” and burn wound infections, has lophotrichous flagella. Flagella that cover the entire surface of a bacterial cell are called peritrichous flagella. The gram-negative bacterium Escherichia coli shows a peritrichous arrangement of flagella.
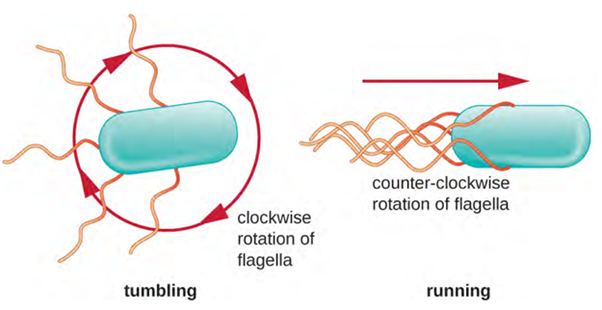
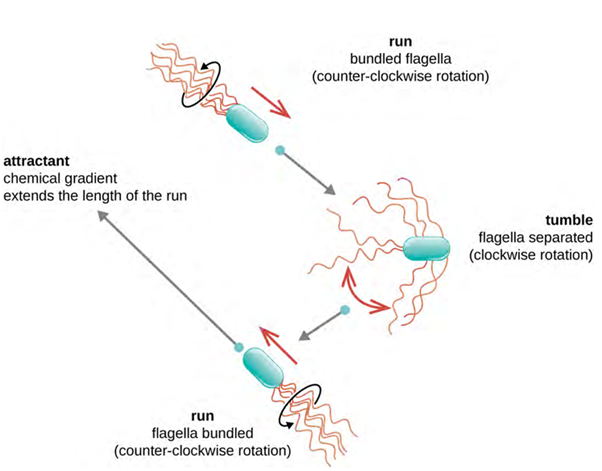
Directional movement depends on the configuration of the flagella. Bacteria can move in response to a variety of environmental signals, including light (phototaxis), magnetic fields (magnetotaxis) using magnetosomes, and, most commonly, chemical gradients (chemotaxis). Purposeful movement toward a chemical attractant, like a food source, or away from a repellent, like a poisonous chemical, is achieved by increasing the length of runs and decreasing the length of tumbles. When running, flagella rotate in a counter clockwise (CCW) direction, allowing the bacterial cell to move forward. In a peritrichous bacterium, the flagella are all bundled together in a very streamlined way (Figure 3.29), allowing for efficient movement. When tumbling, flagella are splayed out while rotating in a clockwise direction, creating a looping motion, and preventing meaningful forward movement but reorienting the cell toward the direction of the attractant. When an attractant exists, runs and tumbles still occur; however, the length of runs is longer, while the length of the tumbles is reduced, allowing overall movement toward the higher concentration of the attractant. When no chemical gradient exists, the lengths of runs and tumbles are more equal, and overall movement is more random (Figure 3.30).
The stimuli that ‘inform’ these movements are detected by protein receptors embedded within their membrane, called chemoreceptors, which bind specific molecules. Binding typically results in methylation or phosphorylation of the chemoreceptor itself, which triggers an elaborate protein pathway that eventually impacts the rotation of the flagellar motor. The bacteria engage in temporal sensing, where they compare the concentration of a substance with the concentration obtained just a few seconds (or microseconds) earlier. In this way they gather information about the orientation of the concentration gradient of the substance.
Other forms of motility also exist: swimming, corkscrew motility and gliding.
Swimming
Rotation of the flagellar basal body occurs due to the proton motive force, where protons that accumulate on the outside of the cell membrane are driven through pores in the Mot proteins, interacting with charges in the ring proteins as they pass across the membrane. The interaction causes the basal body to rotate and turns the filament extending from the cell. Rotation can occur at 200-1000 rpm and result in speeds of 60 cell lengths/second (for comparison, a cheetah moves at a maximum rate of 25 body lengths/second).
Rotation can occur in a clockwise (CW) or a counter clockwise (CCW) direction, with different results to the cell. A bacterium will move forward, called a “run,” when there is a CCW rotation and reorient randomly, called a “tumble,” when there is a CW rotation.
Corkscrew Motility
Some spiral-shaped bacteria, the Spirochetes, use a corkscrew-motility due to their unusual morphology and flagellar conformation. These gram-negative bacteria have specialised flagella that attach to one end of the cell, extend back through the periplasm and then attach to the other end of the cell. When these endoflagella (internal flagella) rotate they put torsion on the entire cell, resulting in a flexing motion that is particularly effective for burrowing through viscous liquids.
Gliding Motility
Gliding motility is just like it sounds, a slower and more graceful movement than the other forms covered so far. Gliding motility is exhibited by certain filamentous or bacillus bacteria and does not require the use of flagella. It does require that the cells be in contact with a solid surface, although more than one mechanism has been identified. Some cells rely on slime propulsion, where secreted slime propels the cell forward, where other cells rely on surface layer proteins to pull the cell forward.
Eukaryotic Cells
Some eukaryotic cells use flagella for locomotion; however, eukaryotic flagella are structurally distinct from those found in prokaryotic cells. Whereas the prokaryotic flagellum is a stiff, rotating structure, a eukaryotic flagellum is more like a flexible whip composed of nine parallel pairs of microtubules surrounding a central pair of microtubules. This arrangement is referred to as a 9+2 array (Figure 3.26). The parallel microtubules use dynein motor proteins to move relative to each other, causing the flagellum to bend.
Cilia (singular: cilium) are a similar external structure found in some eukaryotic cells. Unique to eukaryotes, cilia are shorter than flagella and often cover the entire surface of a cell; however, they are structurally similar to flagella (a 9+2 array of microtubules) and use the same mechanism for movement. A structure called a basal body is found at the base of each cilium and flagellum. The basal body, which attaches the cilium or flagellum to the cell, is composed of an array of triplet microtubules similar to that of a centriole but embedded in the plasma membrane. Because of their shorter length, cilia use a rapid, flexible, waving motion. In addition to motility, cilia may have other functions such as sweeping particles past or into cells. For example, ciliated protozoans use the sweeping of cilia to move food particles into their mouthparts, and ciliated cells in the mammalian respiratory tract beat in synchrony to sweep mucus and debris up and out of the lungs (Figure 3.31).
Metabolism
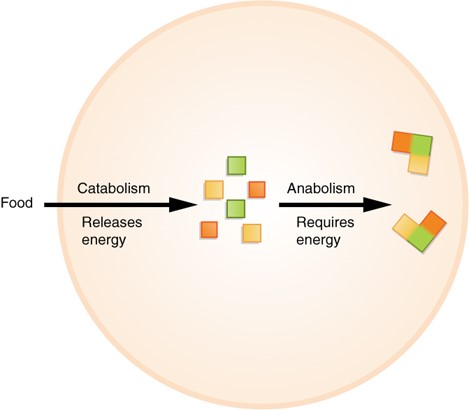
The first law of thermodynamics holds that energy can neither be created nor destroyed—it can only change form. The basic function of an organism is to consume (ingest) energy and molecules and to convert this energy into fuel for movement, sustenance for body functions and to building and maintaining body structures. There are two types of reactions that accomplish this: anabolism and catabolism.
- Anabolism is the process whereby smaller, simpler molecules are combined into larger, more complex substances. The body can assemble, by utilising energy, the complex chemicals it needs by combining small molecules derived from the foods ingested
- Catabolism is the process by which larger more complex substances are broken down into smaller simpler molecules. Catabolism releases energy. The complex molecules found in foods are broken down so the body can use their parts to assemble the structures and substances needed for life.
Taken together, these two processes are called metabolism. Metabolism is the sum of all anabolic and catabolic reactions that take place in the body. Both anabolism and catabolism occur simultaneously and continuously to keep cells (and the body) alive (Figure 3.32).
The energy currency in each cell is a chemical compound, adenosine triphosphate (ATP), which stores and release energy. The cell stores energy in the synthesis (anabolism) of ATP, then moves the ATP molecules to the location where energy is needed to fuel cellular activities. Then the ATP is broken down (catabolism) and a controlled amount of energy is released, which is used by the cell to perform a particular job.
Prokaryotes produce ATP by a special proton pump gradient located along the cell membrane whereas eukaryotes have mitochondria where ATP is produced.
Extracellular Matrix
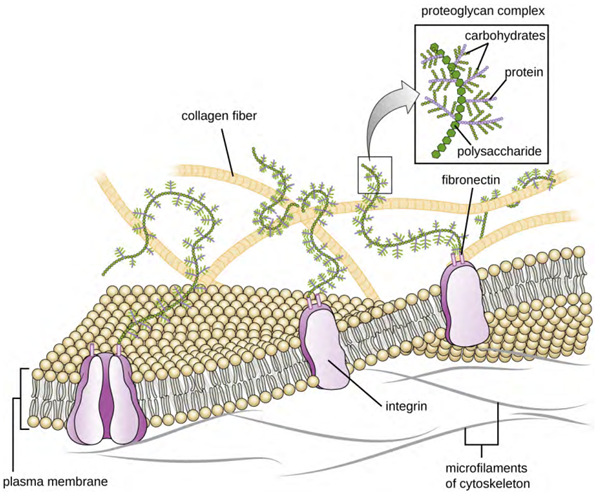
Cells of animals and some protozoans do not have cell walls to help maintain shape and provide structural stability. Instead, these types of eukaryotic cells produce an extracellular matrix (ECM) for this purpose. They secrete a sticky mass of carbohydrates and proteins into the spaces between adjacent cells (Figure 3.33). Some protein components assemble into a basement membrane to which the remaining extracellular matrix components adhere. Proteoglycans typically form the bulky mass of the extracellular matrix while fibrous proteins, like collagen, provide strength. Both proteoglycans and collagen are attached to fibronectin proteins, which, in turn, are attached to integrin proteins. These integrin proteins interact with transmembrane proteins in the plasma membranes of eukaryotic cells that lack cell walls.
Archaea and bacteria
Found in eukaryotic cells and contains the DNA genome.
‘Deoxyribonucleic acid’. Contains genetic information for cell function, growth and division.
Full complement of DNA within a cell organised into smaller, discrete units called genes, arranged on chromosomes and plasmids.
Made of organised and packaged DNA in the form of genes and are found within the cell nucleus.
Small, circular, double-stranded DNA molecules, often found in bacteria, less common in archaea and eukaryotic organisms.
Functional length of DNA that provides the genetic information necessary to build a protein
Site of protein synthesis. Converts mRNA into amino acid chain that is folded into a protein and further processed in the Golgi apparatus.
Biological macromolecule comprised of one or more amino acid chains.
Plants, animals, protozoans, algae and fungi.
Full complement of DNA within a cell organised into smaller, discrete units called genes, arranged on chromosomes and plasmids.
Different forms of a characteristic.
Attachment of a covalently bonded hydrogen atom to another atom.
Ribonucleic acid, similar in structure to DNA. Single stranded and shorter than DNA, RNA carries genetic information to ribosomes to be translated into proteins.
Membrane-bound organelle found in most eukaryotic cells, often considered the control center of the cell because it houses the cell's genetic material, DNA (deoxyribonucleic acid).
Site of protein synthesis. Converts mRNA into amino acid chain that is folded into a protein and further processed in the Golgi apparatus.
Gel-like substance composed of water and dissolved chemicals.
Complex sugar and amino acid polymer that forms a tough, protective mesh-like structure in the cell walls of bacteria, providing structural integrity and shape.
Gel-like substance composed of water and dissolved chemicals.
Form part of the cytoskeleton to provide support and are composed of beta and alpha tubulin.
Form part of the cytoskeleton to provide support and are composed of beta and alpha tubulin.
Coenzyme found in all lifeforms, that is important for metabolism as an energy molecule.
Structural elements of animal cells comprised of anti-parallel dimers.
Complex nuclear membrane that surrounds the nucleus, consisting of two distinct lipid bilayers contiguous with each other.
Selectively permeable barrier that separates the interior of a cell from its external environment.
Macromolecule that is nonpolar and insoluble in water.
Biological macromolecule comprised of one or more amino acid chains.
Plants, animals, protozoans, algae and fungi.
Biological macromolecule comprised of one or more amino acid chains.
Biological macromolecule in which the ratio of carbon to hydrogen and to oxygen is 1:2:1; carbohydrates serve as energy sources and structural support in cells and form arthropods' cellular exoskeleton.
PLFA analysis estimates the total biomass of microbiota community composition by analysing cell membrane fatty acids.
Selectively permeable barrier that separates the interior of a cell from its external environment.
Movement of molecules along the concentration gradient.
Movement of molecules along the concentration gradient that does not require energy.
Movement of water across a semipermeable membrane from areas of low concentration to areas of high concentration.
Ability of a surrounding solution to cause a cell to gain or lose water
Archaea and bacteria.
Diffusion of molecules across the plasma membrane with assistance from membrane proteins.
Uses energy to move molecules against the concentration gradient (area of lower concentration to area of higher concentration).
Absorption of materials into a cell.
Immune cell that surrounds, ingests and destroys foreign material.
Molecule that irreversibly binds with a receptor protein molecule.
Secretory vesicles release the contents to the cell’s exterior.
Large, complex molecules that play critical roles in the body, such as assisting in metabolism, transport, stimulation, and cellular replication.
Archaea and bacteria.
Structures used by cells to move in aqueous environments – propeller-like.
Singular flagellum typically located at one end of the cell.
Flagellum or tufts of flagellum at each end of the cell.
Flagella with a tuft at one end of the cell.
Flagella that cover the entire surface of a bacteria cell.
Specialised sensory receptors that convert chemical signals in the environment.
Structures used by cells to move in aqueous environments – propeller-like.
Subatomic particle with positive electrical charge, present in the nuclei of all atoms and slightly lower mass of a neutron.
Science investigating the size, shape and structure of organisms
Hair-like organelle found in large numbers on some cells allowing movement or propulsion.
Simpler molecules are combined into more complex substances.
More complex substances are broken down into simpler molecules.

